Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer
- PMID: 19012856
- PMCID: PMC5600479
- DOI: 10.1016/S1470-2045(08)70277-8
Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer
Abstract
Knowledge of mucins and their multiple roles in various normal and pathological processes has improved greatly in the past two decades. Mucins belong to a family of glycoproteins characterised by densely O-glycosylated repetitive domains and expressed by various surface epithelial cells. Altered expression of mucins is present in various diseases, including cancer. Ovarian cancer is the sixth most common cancer worldwide and the seventh leading cause of cancer-related deaths in women. The most common ovarian cancer is epithelial ovarian carcinoma, which is characterised by few early symptoms, widespread peritoneal dissemination, and ascites at advanced stages that result in poor prognosis. After diagnosis, 5 year survival is only 35-45%. Therefore, improved strategies for early diagnosis and treatment are needed. Because of the surface epithelial origin of epithelial ovarian cancer, mucins are obvious biomolecules for investigation as markers for early diagnosis and as therapeutic targets. We discuss the potential role and clinical usefulness of mucins in early diagnosis, prognosis, and treatment of ovarian cancer.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
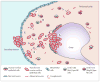
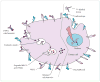

References
-
- Sun CC, Ramirez PT, Bodurka DC. Quality of life for patients with epithelial ovarian cancer. Nat Clin Pract Oncol. 2007;4:18–29. - PubMed
-
- Hankinson SE, Colditz GA, Hunter DJ, et al. A prospective study of reproductive factors and risk of epithelial ovarian cancer. Cancer. 1995;76:284–90. - PubMed
-
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22:255–88. - PubMed
-
- Lynch HT, Casey MJ, Shaw TG, Lynch JF. Hereditary factors in gynecologic cancer. Oncologist. 1998;3:319–38. - PubMed
-
- Auersperg N, Edelson MI, Mok SC, Johnson SW, Hamilton TC. The biology of ovarian cancer. Semin Oncol. 1998;25:281–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

