Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial
- PMID: 19012954
- PMCID: PMC2721012
- DOI: 10.1016/S0140-6736(08)61591-3
Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial
Abstract
Background: Observational data and non-human primate challenge studies suggest that cell-mediated immune responses might provide control of HIV replication. The Step Study directly assessed the efficacy of a cell-mediated immunity vaccine to protect against HIV-1 infection or change in early plasma HIV-1 levels.
Methods: We undertook a double-blind, phase II, test-of-concept study at 34 sites in North America, the Caribbean, South America, and Australia. We randomly assigned 3000 HIV-1-seronegative participants by computer-generated assignments to receive three injections of MRKAd5 HIV-1 gag/pol/nef vaccine (n=1494) or placebo (n=1506). Randomisation was prestratified by sex, adenovirus type 5 (Ad5) antibody titre at baseline, and study site. Primary objective was a reduction in HIV-1 acquisition rates (tested every 6 months) or a decrease in HIV-1 viral-load setpoint (early plasma HIV-1 RNA measured 3 months after HIV-1 diagnosis). Analyses were per protocol and modified intention to treat. The study was stopped early because it unexpectedly met the prespecified futility boundaries at the first interim analysis. This study is registered with ClinicalTrials.gov, number NCT00095576.
Findings: In a prespecified interim analysis in participants with baseline Ad5 antibody titre 200 or less, 24 (3%) of 741 vaccine recipients became HIV-1 infected versus 21 (3%) of 762 placebo recipients (hazard ratio [HR] 1.2 [95% CI 0.6-2.2]). All but one infection occurred in men. The corresponding geometric mean plasma HIV-1 RNA was comparable in infected male vaccine and placebo recipients (4.61 vs 4.41 log(10) copies per mL, one tailed p value for potential benefit 0.66). The vaccine elicited interferon-gamma ELISPOT responses in 75% (267) of the 25% random sample of all vaccine recipients (including both low and high Ad5 antibody titres) on whose specimens this testing was done (n=354). In exploratory analyses of all study volunteers, irrespective of baseline Ad5 antibody titre, the HR of HIV-1 infection between vaccine and placebo recipients was higher in Ad5 seropositive men (HR 2.3 [95% CI 1.2-4.3]) and uncircumcised men (3.8 [1.5-9.3]), but was not increased in Ad5 seronegative (1.0 [0.5-1.9]) or circumcised (1.0 [0.6-1.7]) men.
Interpretation: This cell-mediated immunity vaccine did not prevent HIV-1 infection or reduce early viral level. Mechanisms for insufficient efficacy of the vaccine and the increased HIV-1 infection rates in subgroups of vaccine recipients are being explored.
Figures
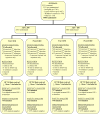
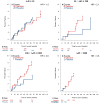


Comment in
-
Failure of the Merck HIV vaccine: an uncertain step forward.Lancet. 2008 Nov 29;372(9653):1857-1858. doi: 10.1016/S0140-6736(08)61593-7. Epub 2008 Nov 13. Lancet. 2008. PMID: 19012958 No abstract available.
-
HIV-1 step study.Lancet. 2009 Mar 7;373(9666):805; author reply 806. doi: 10.1016/S0140-6736(09)60469-4. Lancet. 2009. PMID: 19269505 No abstract available.
-
HIV-1 step study.Lancet. 2009 Mar 7;373(9666):805-6; author reply 806. doi: 10.1016/S0140-6736(09)60471-2. Lancet. 2009. PMID: 19269506 No abstract available.
-
HIV-1 step study.Lancet. 2009 Mar 7;373(9666):805; author reply 806. doi: 10.1016/S0140-6736(09)60470-0. Lancet. 2009. PMID: 19269507 No abstract available.
-
Immunology. Immune activation with HIV vaccines.Science. 2014 Apr 4;344(6179):49-51. doi: 10.1126/science.1250672. Science. 2014. PMID: 24700849 Free PMC article. No abstract available.
References
-
- Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006 Dec;6(12):930–9. - PubMed
-
- Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis. 2006 Aug 15;43:500–11. - PubMed
-
- Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004 Mar;5(3):233–6. - PubMed
-
- Haynes BF, Montefiori DC. Aiming to induce broadly reactive neutralizing antibody responses with HIV-1 vaccine candidates. Expert Rev Vaccines. 2006 Aug;5(4):579–95. - PubMed
-
- Polonis VR, Brown BK, Rosa BA, Zolla-Pazner S, Dimitrov DS, Zhang MY, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008 Jun 5;375(2):315–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

