The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage
- PMID: 19013146
- PMCID: PMC2634829
- DOI: 10.1016/j.ydbio.2008.10.025
The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage
Abstract
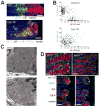
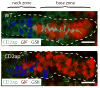
In the mammalian gastrointestinal tract, the cell fate decisions that specify the development of multiple, diverse lineages are governed in large part by interactions of stem and early lineage progenitor cells with their microenvironment, or niche. Here, we show that the gastric parietal cell (PC) is a key cellular component of the previously undescribed niche for the gastric epithelial neck cell, the progenitor of the digestive enzyme secreting zymogenic (chief) cell (ZC). Genetic ablation of PCs led to failed patterning of the entire zymogenic lineage: progenitors showed premature expression of differentiated cell markers, and fully differentiated ZCs failed to develop. We developed a separate mouse model in which PCs localized not only to the progenitor niche, but also ectopically to the gastric unit base, which is normally occupied by terminally differentiated ZCs. Surprisingly, these mislocalized PCs did not maintain adjacent zymogenic lineage cells in the progenitor state, demonstrating that PCs, though necessary, are not sufficient to define the progenitor niche. We induced this PC mislocalization by knocking out the cytoskeleton-regulating gene Cd2ap in Mist1(-/-) mice, which led to aberrant E-cadherin localization in ZCs, irregular ZC-ZC junctions, and disruption of the ZC monolayer by PCs. Thus, the characteristic histology of the gastric unit, with PCs in the middle and ZCs in the base, may depend on establishment of an ordered adherens junction network in ZCs as they migrate into the base.
Figures








References
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. - PubMed
-
- Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. - PubMed
-
- Blostein R, Zhang R, Gottardi CJ, Caplan MJ. Functional properties of an H,K-ATPase/Na,K-ATPase chimera. J Biol Chem. 1993;268:10654–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

