Posttraumatic epilepsy after controlled cortical impact injury in mice
- PMID: 19013458
- PMCID: PMC4694635
- DOI: 10.1016/j.expneurol.2008.10.005
Posttraumatic epilepsy after controlled cortical impact injury in mice
Abstract
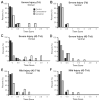
Many patients develop temporal lobe epilepsy after trauma, but basic mechanisms underlying the development of chronic seizures after head injury remain poorly understood. Using the controlled cortical impact injury model we examined whether mice developed spontaneous seizures after mild (0.5 mm injury depth) or severe (1.0 mm injury depth) brain injury and how subsequent posttraumatic mossy fiber sprouting was associated with excitability in the dentate gyrus 42-71 d after injury. After several weeks, spontaneous behavioral seizures were observed in 20% of mice with mild and 36% of mice with severe injury. Mossy fiber sprouting was typically present in septal slices of the dentate gyrus ipsilateral to the injury, but not in control mice. In slices with mossy fiber sprouting, perforant path stimulation revealed a significant reduction (P<0.01) in paired-pulse ratios in dentate granule cells at 20 ms and 40 ms interpulse intervals, but not at 80 ms or 160 ms intervals. These slices were also characterized by spontaneous and hilar-evoked epileptiform activity in the dentate gyrus in the presence of Mg(2+)-free ACSF containing 100 microM picrotoxin. In contrast, paired-pulse and hilar-evoked responses in slices from injured animals that did not display mossy fiber sprouting were not different from controls. These data suggest the development of spontaneous posttraumatic seizures as well as structural and functional network changes associated with temporal lobe epilepsy in the mouse dentate gyrus by 71 d after CCI injury. Identifying experimental injury models that exhibit similar pathology to injury-induced epilepsy in humans should help to elucidate the mechanisms by which the injured brain becomes epileptic.
Figures



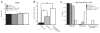

References
-
- Anderson KJ, Miller KM, Fugaccia I, Scheff SW. Regional distribution of Fluoro-Jade B staining in the hippocampus following traumatic brain injury. Exp Neurol. 2005;193:125–130. - PubMed
-
- Anderson RW, Brown CJ, Blumbers PC, McLean AJ, Jones NR. Impact mechanics and axonal injury in a sheep model. J Neurotrauma. 2003;20:961–974. - PubMed
-
- Annegers JF, Hauser A, Coan SP, Rocca WP. A population-based study of seizures and traumatic brain injuries. N Eng J Med. 1998;338:20–24. - PubMed
-
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neurosci. 1985;14:375–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

