Requirements for cell rounding and surface protein down-regulation by Ebola virus glycoprotein
- PMID: 19013626
- PMCID: PMC2654768
- DOI: 10.1016/j.virol.2008.10.029
Requirements for cell rounding and surface protein down-regulation by Ebola virus glycoprotein
Abstract
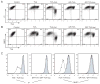
Ebola virus causes an acute hemorrhagic fever that is associated with high morbidity and mortality. The viral glycoprotein is thought to contribute to pathogenesis, though precise mechanisms are unknown. Cellular pathogenesis can be modeled in vitro by expression of the Ebola viral glycoprotein (GP) in cells, which causes dramatic morphological changes, including cell rounding and surface protein down-regulation. These effects are known to be dependent on the presence of a highly glycosylated region of the glycoprotein, the mucin domain. Here we show that the mucin domain from the highly pathogenic Zaire subtype of Ebola virus is sufficient to cause characteristic cytopathology when expressed in the context of a foreign glycoprotein. Similarly to full length Ebola GP, expression of the mucin domain causes rounding, detachment from the extracellular matrix, and the down-regulation of cell surface levels of beta1 integrin and major histocompatibility complex class 1. These effects were not seen when the mucin domain was expressed in the context of a glycophosphatidylinositol-anchored isoform of the foreign glycoprotein. In contrast to earlier analysis of full length Ebola glycoproteins, chimeras carrying the mucin domains from the Zaire and Reston strains appear to cause similar levels of down-modulation and cell detachment. Cytopathology associated with Ebola glycoprotein expression does not occur when GP expression is restricted to the endoplasmic reticulum. In contrast to a previously published report, our results demonstrate that GP-induced surface protein down-regulation is not mediated through a dynamin-dependent pathway. Overall, these results support a model in which the mucin domain of Ebola GP acts at the cell surface to induce protein down modulation and cytopathic effects.
Figures


References
-
- Alazard-Dany N, Volchkova V, Reynard O, Carbonnelle C, Dolnik O, Ottmann M, Khromykh A, Volchkov VE. Ebola virus glycoprotein GP is not cytotoxic when expressed constitutively at a moderate level. J Gen Virol. 2006;87(Pt 5):1247–57. - PubMed
-
- Barrientos LG, Rollin PE. Release of cellular proteases into the acidic extracellular milieu exacerbates Ebola virus-induced cell damage. Virology 2006 - PubMed
-
- Bates P, Young JA, Varmus HE. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell. 1993;74(6):1043–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

