Change in markers of bone metabolism with chemotherapy for advanced prostate cancer: interleukin-6 response is a potential early indicator of response to therapy
- PMID: 19014338
- PMCID: PMC2956618
- DOI: 10.1089/jir.2008.0024
Change in markers of bone metabolism with chemotherapy for advanced prostate cancer: interleukin-6 response is a potential early indicator of response to therapy
Abstract
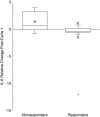
Men with androgen-independent prostate cancer (AIPC) frequently have bone metastasis. The effects of chemotherapy on markers of bone metabolism have not been well characterized. We conducted a prospective study of patients with AIPC randomized in the first cycle to receive either docetaxel/estramustine or zoledronic acid, a bisphosphonate, to inhibit osteoclastic activity. Here we report the effects of therapy on markers of bone metabolism in these patients following the first cycle of therapy. Serum levels of several indices of bone remodeling were evaluated using commercial enzyme-linked immunosorbent assays. Changes in markers of bone metabolism were compared in patients receiving initial chemotherapy versus bisphosphonate. There was no significant difference in median change in any of the measured bone markers in patients given zoledronic acid when compared to chemotherapy. When comparing responders to nonresponders, overall interleukin-6 (IL-6) decreased by 35% in prostate-specific antigen responders; whereas, IL-6 levels increased by 76% in nonresponders (p = 0.03). Elevated IL-6 levels and reductions in IL-6 levels early in treatment may reflect ultimate clinical response to docetaxel-based regimens.
Figures

References
-
- Akimoto S. Okumura A. Fuse H. Relationship between serum levels of interleukin-6, tumor necrosis factor-alpha and bone turnover markers in prostate cancer patients. Endocr J. 1998;45(2):183–189. - PubMed
-
- Berenson J. Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist. 2005;10:52–62. - PubMed
-
- Brown J. Corey E. Lee Z. True LD. Yun TJ. Tondravi M. Vessela RL. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57:611–616. - PubMed
-
- Bubley G. Carducci M. Dahut W. Dawson N. Daliani D. Eisenberger M. Figg W. Freidlin B. Halabi S. Hudes G. Hussain M. Kaplan R. Myers C. Oh W. Petrylak D. Reed E. Roth B. Sartor O. Scher H. Simons J. Sinibaldi V. Small E. Smith M. Trump D. Wilding G. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17(11):3461–3467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

