IL-1beta differently involved in IL-8 and FGF-2 release in crystalline silica-treated lung cell co-cultures
- PMID: 19014534
- PMCID: PMC2588635
- DOI: 10.1186/1743-8977-5-16
IL-1beta differently involved in IL-8 and FGF-2 release in crystalline silica-treated lung cell co-cultures
Abstract
Background: Inhalation of crystalline silica particles is in humans associated with inflammation and development of fibrosis. The aim of the present study was to investigate the effect of crystalline silica on the release of the fibrosis- and angiogenesis-related mediator FGF-2 and the pro-inflammatory mediator IL-8, and how IL-1beta and TNF-alpha were involved in this release from various mono- and co-cultures of monocytes, pneumocytes and endothelial cells.
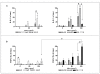
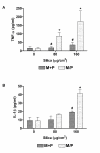
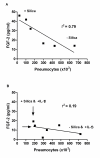
Results: Silica exposure induced an increase of IL-8 release from monocytes and from pneumocytes alone, and the FGF-2 level in the medium increased upon silica exposure of pneumocytes. Both the responses were enhanced in non-contact co-cultures with endothelial cells. The FGF-2 release seemed to increase with the silica-induced decrease in the number of pneumocytes. The release of IL-8 and FGF-2 was partially suppressed in cultures with pneumocytes in contact with monocytes compared to non-contact cultures. Treatment with anti-TNF-alpha and the IL-1 receptor antagonist revealed that release of IL-1beta, and not TNF-alpha, from monocytes dominated the regulation of IL-8 release in co-cultures. For release of FGF-2, IL-1ra was without effect. However, exogenous IL-1beta reduced the FGF-2 levels, strongly elevated the FGF-2-binding protein PTX3, and prevented the reduction in the number of pneumocytes induced by silica.
Conclusion: IL-1beta seems to be differently involved in the silica-induced release of IL-8 and FGF-2 in different lung cell cultures. Whereas the silica-induced IL-8 release is regulated via an IL-1-receptor-mediated mechanism, IL-1beta is suggested only indirectly to affect the silica-induced FGF-2 release by counteracting pneumocyte loss. Furthermore, the enhanced IL-8 and FGF-2 responses in co-cultures involving endothelial cells show the importance of the interaction between different cell types and may suggest that both these mediators are important in angiogenic or fibrogenic processes.
Figures



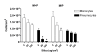


References
-
- Davis GS, Pfeiffer LM, Hemenway DR. Persistent overexpression of interleukin-1beta and tumor necrosis factor-alpha in murine silicosis. J Environ Pathol Toxicol Oncol. 1998;17:99–114. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

