One-year treatment with mometasone furoate in chronic obstructive pulmonary disease
- PMID: 19014549
- PMCID: PMC2644301
- DOI: 10.1186/1465-9921-9-73
One-year treatment with mometasone furoate in chronic obstructive pulmonary disease
Abstract
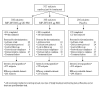
Many patients with chronic obstructive pulmonary disease (COPD) are treated with twice daily (BID) inhaled corticosteroids (ICS). This study evaluated whether daily PM mometasone furoate administered via a dry powder inhaler (MF-DPI) was equally effective compared to twice daily dosing.In a 52-week, randomized, double-blind, placebo-controlled study, 911 subjects with moderate-to-severe COPD managed without ICS received MF-DPI 800 microg QD PM, MF-DPI 400 microg BID, or placebo. The change from baseline in postbronchodilator forced expiratory volume in 1 second (FEV1), total COPD symptom scores, and health status as well as the percentage of subjects with a COPD exacerbation were assessed. Adverse events were recorded. Mometasone furoate administered via a dry powder inhaler 800 microg QD PM and 400 microg BID significantly increased postbronchodilator FEV1 from baseline (50 mL and 53 mL, respectively, versus a 19 mL decrease for placebo; P < 0.001). The percentage of subjects exacerbating was significantly lower in the pooled MF-DPI groups than in the placebo group (P = 0.043). Subjects receiving MF-DPI 400 microg BID reported a statistically significant (19%) reduction in COPD symptom scores compared with placebo (P < 0.001). Health status as measured with St. George's Respiratory Questionnaire (SGRQ) improved significantly in all domains (Total, Activity, Impacts, and Symptoms) in the pooled MF-DPI groups versus placebo (P < or = 0.031). MF-DPI treatment was well tolerated.Once-daily MF-DPI improved lung function and health status in subjects with moderate-to-severe COPD and was comparable to BID MF-DPI.
Figures

References
-
- Chartbook on Cardiovascular, Lung, and Blood Diseases http://www.nhlbi.nih.gov/resources/docs/cht-book.htm
-
- Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled corticosteroids. New developments. Am J Respir Crit Care Med. 1998;157(3 Pt 2):S1–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

