The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ
- PMID: 19014551
- PMCID: PMC2592246
- DOI: 10.1186/1471-2202-9-112
The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ
Abstract
Background: The Kv2.1 delayed-rectifier K+ channel regulates membrane excitability in hippocampal neurons where it targets to dynamic cell surface clusters on the soma and proximal dendrites. In the past, Kv2.1 has been assumed to be absent from the axon initial segment.
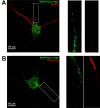
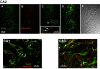
Results: Transfected and endogenous Kv2.1 is now demonstrated to preferentially accumulate within the axon initial segment (AIS) over other neurite processes; 87% of 14 DIV hippocampal neurons show endogenous channel concentrated at the AIS relative to the soma and proximal dendrites. In contrast to the localization observed in pyramidal cells, GAD positive inhibitory neurons within the hippocampal cultures did not show AIS targeting. Photoactivable-GFP-Kv2.1-containing clusters at the AIS were stable, moving <1 microm/hr with no channel turnover. Photobleach studies indicated individual channels within the cluster perimeter were highly mobile (FRAP tau=10.4+/-4.8 sec), supporting our model that Kv2.1 clusters are formed by the retention of mobile channels behind a diffusion-limiting perimeter. Demonstrating that the AIS targeting is not a tissue culture artifact, Kv2.1 was found in axon initial segments within both the adult rat hippocampal CA1, CA2, and CA3 layers and cortex.
Conclusion: In summary, Kv2.1 is associated with the axon initial segment both in vitro and in vivo where it may modulate action potential frequency and back propagation. Since transfected Kv2.1 initially localizes to the AIS before appearing on the soma, it is likely multiple mechanisms regulate Kv2.1 trafficking to the cell surface.
Figures






References
-
- Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience. 1998;84:37–48. doi: 10.1016/S0306-4522(97)00519-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

