Connective tissue growth factor promoter activity in normal and wounded skin
- PMID: 19014648
- PMCID: PMC2584011
- DOI: 10.1186/1755-1536-1-3
Connective tissue growth factor promoter activity in normal and wounded skin
Abstract
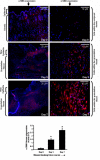
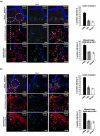
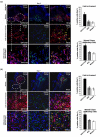
In skin, connective tissue growth factor (CTGF/CCN2) is induced during tissue repair. However, what the exact cell types are that express CTGF in normal and wounded skin remain controversial. In this report, we use transgenic knock-in mice in which the Pacific jellyfish Aequorea victoria enhanced green fluorescent protein (E-GFP) gene has been inserted between the endogenous CTGF promoter and gene. Unwounded (day 0) and wounded (days 3 and 7) skin was examined for GFP to detect cells in which the CTGF promoter was active, alpha-smooth muscle actin (alpha-SMA) to detect myofibroblasts, and NG2 expression to detect pericytes. In unwounded mice, CTGF expression was absent in epidermis and was present in a few cells in the dermis. Upon wounding, CTGF expression was induced in the dermis. Double immunolabeling revealed that CTGF-expressing cells also expressed alpha-SMA, indicating the CTGF was expressed in myofibroblasts. A subset (approximately 30%) of myofibroblasts were also NG2 positive, indicating that pericytes significantly contributed to the number of myofibroblasts in the wound. Pericytes also expressed CTGF. Collectively, these results indicate that CTGF expression in skin correlates with myofibroblast induction, and that CTGF-expressing pericytes are significant contributors to myofibroblast activity during cutaneous tissue repair.
Figures


Similar articles
-
Connective tissue growth factor is induced in bleomycin-induced skin scleroderma.J Cell Commun Signal. 2010 Mar;4(1):25-30. doi: 10.1007/s12079-009-0081-3. Epub 2009 Nov 15. J Cell Commun Signal. 2010. PMID: 19916059 Free PMC article.
-
Smad7 blocks transforming growth factor-β1-induced gingival fibroblast-myofibroblast transition via inhibitory regulation of Smad2 and connective tissue growth factor.J Periodontol. 2011 Apr;82(4):642-51. doi: 10.1902/jop.2010.100510. Epub 2010 Nov 8. J Periodontol. 2011. PMID: 21054221
-
Pericytes display increased CCN2 expression upon culturing.J Cell Commun Signal. 2009 Mar;3(1):61-4. doi: 10.1007/s12079-009-0053-7. Epub 2009 Apr 22. J Cell Commun Signal. 2009. PMID: 19384472 Free PMC article.
-
Immunophenotypical analyses of myofibroblasts in rat excisional wound healing: possible transdifferentiation of blood vessel pericytes and perifollicular dermal sheath cells into myofibroblasts.Histol Histopathol. 2012 Apr;27(4):515-27. doi: 10.14670/HH-27.515. Histol Histopathol. 2012. PMID: 22374729
-
Role of CTGF gene promoter methylation in the development of hepatic fibrosis.Am J Transl Res. 2016 Jan 15;8(1):125-32. eCollection 2016. Am J Transl Res. 2016. PMID: 27069546 Free PMC article.
Cited by
-
Getting out of a sticky situation: targeting the myofibroblast in scleroderma.Open Rheumatol J. 2012;6:163-9. doi: 10.2174/1874312901206010163. Epub 2012 Jun 15. Open Rheumatol J. 2012. PMID: 22802915 Free PMC article.
-
CCN2: a mechanosignaling sensor modulating integrin-dependent connective tissue remodeling in fibroblasts?J Cell Commun Signal. 2013 Aug;7(3):203-5. doi: 10.1007/s12079-013-0205-7. J Cell Commun Signal. 2013. PMID: 23729366 Free PMC article.
-
mPGES-1 null mice are resistant to bleomycin-induced skin fibrosis.Arthritis Res Ther. 2011;13(1):R6. doi: 10.1186/ar3226. Epub 2011 Jan 25. Arthritis Res Ther. 2011. PMID: 21266028 Free PMC article.
-
Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins.Am J Pathol. 2013 Nov;183(5):1559-1570. doi: 10.1016/j.ajpath.2013.07.016. Epub 2013 Sep 5. Am J Pathol. 2013. PMID: 24012677 Free PMC article.
-
Conditional knockout of CTGF affects corneal wound healing.Invest Ophthalmol Vis Sci. 2014 Apr 1;55(4):2062-70. doi: 10.1167/iovs.13-12735. Invest Ophthalmol Vis Sci. 2014. PMID: 24627144 Free PMC article.
References
-
- Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–546. - PubMed
-
- Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. - PubMed
-
- Chen Y, Shi-wen X, van Beek J, Kennedy L, McLeod M, Renzoni EA, Bou-Gharios G, Wilcox-Adelman S, Goetinck PF, Eastwood M, Black CM, Abraham DJ, Leask A. Matrix contraction by dermal fibroblasts requires transforming growth factor-beta/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am J Pathol. 2005;67:1699–1711. - PMC - PubMed
-
- Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. - PubMed
-
- Rajkumar VS, Shi-wen X, Bostrom M, Leoni P, Muddle J, Ivarsson M, Gerdin B, Denton CP, Bou-Gharios G, Black CM, Abraham DJ. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–2265. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

