Smad4 haploinsufficiency: a matter of dosage
- PMID: 19014666
- PMCID: PMC2580039
- DOI: 10.1186/1755-8417-1-2
Smad4 haploinsufficiency: a matter of dosage
Abstract
Background: The inactivation of tumor suppressor genes follows Alfred Knudson's 'two-hit' model: both alleles need to be inactivated by independent mutation events to trigger tumor formation. However, in a minority of tumor suppressor genes a single hit is sufficient to initiate tumorigenesis notwithstanding the presence of the wild-type allele, a condition known as haploinsufficiency. The SMAD4 gene is an intracellular mediator of the TGF-beta and BMP signal transduction pathways and a tumor suppressor involved in pancreatic and colorectal tumorigenesis. In Smad4-mutant mouse models, haploinsufficiency characterizes the development of gastrointestinal polyps with initial retention of the wild-type allele and protein expression within the nascent tumors and in their direct microenvironment. Similarly, germline SMAD4 mutations are responsible for a subset of patients affected by juvenile polyposis syndrome, an autosomal dominant intestinal cancer syndrome. To date, the molecular and cellular consequences of SMAD4 haploinsufficiency on TGF-beta and BMP signaling and on genome-wide gene expression have not been investigated.
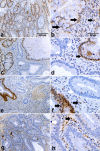
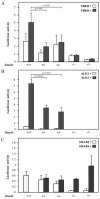
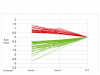
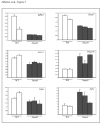
Results: Here we show that, similar to previous observations in Smad4-mutant mouse models, haploinsufficiency characterizes a substantial fraction of the juvenile polyps arising in patients with germline SMAD4 mutations. Also, mouse embryonic and intestinal cells heterozygous for a targeted Smad4 null mutation are characterized by a corresponding 50% reduction of the Smad4 protein levels. Reporter assays revealed that mouse Smad4+/- cells exert intermediate inhibitory effects on both TGF-beta and BMP signaling. Genome-wide expression profiling analysis of Smad4+/- and Smad4-/- cells pinpointed a subset of dosage-dependent transcriptional target genes encompassing, among others, members of the TGF-beta and Wnt signaling pathways. These SMAD4 dosage-dependent transcriptional changes were confirmed and validated in a subset of target genes in intestinal tissues from juvenile polyposis syndrome patients.
Conclusion: Smad4 haploinsufficiency is sufficient to significantly inhibit both TGF-beta and BMP signal transduction and results in the differential expression of a broad subset of target genes likely to underlie tumor formation both from the mesenchymal and epithelial compartments. The results of our study, performed in normal rather than tumor cells where additional (epi-) genetic alterations may confound the analysis, are relevant for our understanding and elucidation of the initial steps underlying SMAD4-driven intestinal tumorigenesis.
Figures

References
-
- Payne SR, Kemp CJ. Tumor suppressor genetics. Carcinogenesis. 2005;26:2031–2045. - PubMed
-
- Fodde R, Smits R. Cancer biology. A matter of dosage. Science. 2002;298:761–763. - PubMed
-
- Howe JR, Roth S, Ringold JC, Summers RW, Jarvinen HJ, Sistonen P, Tomlinson IP, Houlston RS, Bevan S, Mitros FA, Stone EM, Aaltonen LA. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998;280:1086–1088. - PubMed
-
- Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L, Zhou YX, Weinstein M, Kim SJ, Deng CX. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene. 2000;19:1868–1874. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

