PTTG: an important target gene for ovarian cancer therapy
- PMID: 19014669
- PMCID: PMC2584053
- DOI: 10.1186/1757-2215-1-6
PTTG: an important target gene for ovarian cancer therapy
Abstract
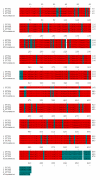
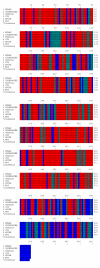
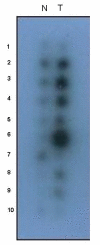
Pituitary tumor transforming gene (PTTG), also known as securin is an important gene involved in many biological functions including inhibition of sister chromatid separation, DNA repair, organ development, and expression and secretion of angiogenic and metastatic factors. Proliferating cancer cells and most tumors express high levels of PTTG. Overexpression of PTTG in vitro induces cellular transformation and development of tumors in nude mice. The PTTG expression levels have been correlated with tumor progression, invasion, and metastasis. Recent studies show that down regulation of PTTG in tumor cell lines and tumors in vivo results in suppression of tumor growth, suggesting its important role in tumorigenesis. In this review, we focus on PTTG structure, sub-cellular distribution, cellular functions, and role in tumor progression with suggestions on possible exploration of this gene for cancer therapy.
Figures



Similar articles
-
Pituitary tumor transforming gene: an important gene in normal cellular functions and tumorigenesis.Histol Histopathol. 2007 Feb;22(2):219-26. doi: 10.14670/HH-22.219. Histol Histopathol. 2007. PMID: 17149695 Review.
-
Development of cystic glandular hyperplasia of the endometrium in Mullerian inhibitory substance type II receptor-pituitary tumor transforming gene transgenic mice.J Endocrinol. 2007 Jul;194(1):179-91. doi: 10.1677/JOE-06-0036. J Endocrinol. 2007. PMID: 17592032
-
Regulation of angiogenesis and invasion by human Pituitary tumor transforming gene (PTTG) through increased expression and secretion of matrix metalloproteinase-2 (MMP-2).Mol Cancer. 2006 Nov 10;5:61. doi: 10.1186/1476-4598-5-61. Mol Cancer. 2006. PMID: 17096843 Free PMC article.
-
Effect of PTTG on endogenous gene expression in HEK 293 cells.BMC Genomics. 2009 Dec 3;10:577. doi: 10.1186/1471-2164-10-577. BMC Genomics. 2009. PMID: 19958546 Free PMC article.
-
Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update.Endocr Relat Cancer. 2008 Sep;15(3):721-43. doi: 10.1677/ERC-08-0012. Endocr Relat Cancer. 2008. PMID: 18753362 Review.
Cited by
-
MiRNA-494 inhibits metastasis of cervical cancer through Pttg1.Tumour Biol. 2015 Sep;36(9):7143-9. doi: 10.1007/s13277-015-3440-0. Epub 2015 Apr 16. Tumour Biol. 2015. Retraction in: Tumour Biol. 2017 Apr 20. doi: 10.1007/s13277-017-5487-6. PMID: 25877755 Retracted.
-
Tumorigenic potential of pituitary tumor transforming gene (PTTG) in vivo investigated using a transgenic mouse model, and effects of cross breeding with p53 (+/-) transgenic mice.BMC Cancer. 2012 Nov 20;12:532. doi: 10.1186/1471-2407-12-532. BMC Cancer. 2012. PMID: 23164239 Free PMC article.
-
Radiation-induced senescence in securin-deficient cancer cells promotes cell invasion involving the IL-6/STAT3 and PDGF-BB/PDGFR pathways.Sci Rep. 2013;3:1675. doi: 10.1038/srep01675. Sci Rep. 2013. PMID: 23591770 Free PMC article.
-
Gene expression profiles in human HepG2 cells treated with extracts of the Tamarindus indica fruit pulp.Genes Nutr. 2010 Dec;5(4):331-41. doi: 10.1007/s12263-010-0187-5. Epub 2010 Oct 19. Genes Nutr. 2010. PMID: 21189869 Free PMC article.
-
Altered expression of securin (Pttg1) and serpina3n in the auditory system of hearing-impaired Tff3-deficient mice.Cell Mol Life Sci. 2011 Aug;68(16):2739-49. doi: 10.1007/s00018-010-0586-1. Epub 2010 Nov 15. Cell Mol Life Sci. 2011. PMID: 21076990 Free PMC article.
References
-
- Bachelier R, Xu X, Li C, Qiao W, Furth PA, Lubet RA, Deng CX. Effect of bilateral oophorectomy on mammary tumor formation in BRCA1 mutant mice. Oncol Rep. 2005;14:1117–20. - PubMed
-
- Hensley H, Quinn BA, Wolf RL, Litwin SL, Mabuchi S, Williams SJ, Williams C, Hamilton TC, Connolly DC. Magnetic Resonance Imaging for Detection and Determination of Tumor Volume in a Genetically Engineered Mouse Model of Ovarian Cancer. Cancer Biol Ther. 2007;6:1717–1725. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

