Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children
- PMID: 19014765
- PMCID: PMC3024439
- DOI: 10.1016/j.jaci.2008.08.021
Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children
Abstract
Background: Asthma exacerbations are a common cause of critical illness in children.
Objective: To determine factors associated with exacerbations in children with persistent asthma.
Methods: Regression modeling was used to identify historical, phenotypic, treatment, and time-dependent factors associated with the occurrence of exacerbations, defined by need for oral corticosteroids or emergency or hospital care in the 48-week Pediatric Asthma Controller Trial study. Children age 6 to 14 years with mild-to-moderate persistent asthma were randomized to receive either fluticasone propionate 100 microg twice daily (FP monotherapy), combination fluticasone 100 microg AM and salmeterol twice daily, or montelukast 5 mg once daily.
Results: Of the 285 participants randomized, 48% had 231 exacerbations. Using a multivariate analysis, which included numerous demographic, pulmonary, and inflammatory parameters, only a history of an asthma exacerbation requiring a systemic corticosteroid in the past year (odds ratio [OR], 2.10; P < .001) was associated with a subsequent exacerbation during the trial. During the trial, treatment with montelukast versus FP monotherapy (OR, 2.00; P = .005), season (spring, fall, or winter vs summer; P < or = .001), and average seasonal 5% reduction in AM peak expiratory flow (OR, 1.21; P = .01) were each associated with exacerbations. Changes in worsening of symptoms, beta-agonist use, and low peak expiratory flow track together before an exacerbation, but have poor positive predictive value of exacerbation.
Conclusion: Children with mild-to-moderate persistent asthma with previous exacerbations are more likely to have a repeat exacerbation despite controller treatment. Inhaled corticosteroids are superior to montelukast at modifying the exacerbation risk. Available physiologic measures and biomarkers and diary card tracking are not reliable predictors of asthma exacerbations.
Figures
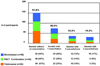
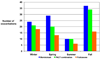
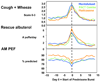
 montelukast,
montelukast,  PACT combination, and
PACT combination, and  FP monotherapy groups, relative to an exacerbation.
FP monotherapy groups, relative to an exacerbation.References
-
- Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–1076. - PubMed
-
- CAMP. Long-term effects of budesonide or nedocromil in children with asthma. Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–1063. - PubMed
-
- Schatz M, Mosen D, Apter AJ, Zeiger RS, Vollmer WM, Stibolt TB, et al. Relationships among quality of life, severity, and control measures in asthma: an evaluation using factor analysis. J Allergy Clin Immunol. 2005;115:1049–1055. - PubMed
-
- Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. J Allergy Clin Immunol. 2007;119:64–72. - PubMed
-
- Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, et al. Risk factors for hospital admission for asthma from childhood to young adulthood: a longitudinal population study. J Allergy Clin Immunol. 2002;110:220–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5U10 HL064288/HL/NHLBI NIH HHS/United States
- M01 RR00051/RR/NCRR NIH HHS/United States
- U10 HL064295/HL/NHLBI NIH HHS/United States
- U10 HL064288/HL/NHLBI NIH HHS/United States
- U10 HL064287/HL/NHLBI NIH HHS/United States
- 5U10 HL064313/HL/NHLBI NIH HHS/United States
- 5U10 HL064295/HL/NHLBI NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- U10 HL064307/HL/NHLBI NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- U10 HL064305/HL/NHLBI NIH HHS/United States
- 5U10 HL064305/HL/NHLBI NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- M01 RR00036/RR/NCRR NIH HHS/United States
- 5U10 HL064307/HL/NHLBI NIH HHS/United States
- 5U10 HL064287/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

