Ethanol exposure decreases mitochondrial outer membrane permeability in cultured rat hepatocytes
- PMID: 19014900
- PMCID: PMC2656607
- DOI: 10.1016/j.abb.2008.10.036
Ethanol exposure decreases mitochondrial outer membrane permeability in cultured rat hepatocytes
Abstract
Mitochondrial metabolism depends on movement of hydrophilic metabolites through the mitochondrial outer membrane via the voltage-dependent anion channel (VDAC). Here we assessed VDAC permeability of intracellular mitochondria in cultured hepatocytes after plasma membrane permeabilization with 8 microM digitonin. Blockade of VDAC with Koenig's polyanion inhibited uncoupled and ADP-stimulated respiration of permeabilized hepatocytes by 33% and 41%, respectively. Tenfold greater digitonin (80 microM) relieved KPA-induced inhibition and also released cytochrome c, signifying mitochondrial outer membrane permeabilization. Acute ethanol exposure also decreased respiration and accessibility of mitochondrial adenylate kinase (AK) of permeabilized hepatocytes membranes by 40% and 32%, respectively. This inhibition was reversed by high digitonin. Outer membrane permeability was independently assessed by confocal microscopy from entrapment of 3 kDa tetramethylrhodamine-conjugated dextran (RhoDex) in mitochondria of mechanically permeabilized hepatocytes. Ethanol decreased RhoDex entrapment in mitochondria by 35% of that observed in control cells. Overall, these results demonstrate that acute ethanol exposure decreases mitochondrial outer membrane permeability most likely by inhibition of VDAC.
Figures


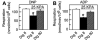

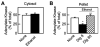


References
-
- Higuchi H, Gores GJ. Curr Mol Med. 2003;3:483–490. - PubMed
-
- Stewart S, Jones D, Day CP. Trends Mol Med. 2001;7:408–413. - PubMed
-
- Tsukamoto H, Lu SC. FASEB J. 2001;15:1335–1349. - PubMed
-
- Bailey SM, Cunningham CC. Free Radic Biol Med. 2002;32:11–16. - PubMed
-
- Cunningham CC, Bailey SM. Biol Signals Recept. 2001;10:271–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

