Copper transport to the brain by the blood-brain barrier and blood-CSF barrier
- PMID: 19014916
- PMCID: PMC2677986
- DOI: 10.1016/j.brainres.2008.10.056
Copper transport to the brain by the blood-brain barrier and blood-CSF barrier
Abstract
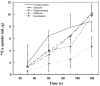
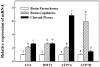
The mechanism of copper (Cu) transport into the brain is unclear. This study evaluated the main species and route of Cu transport into the brain using in situ brain perfusion technique, and assessed the levels of mRNA encoding Cu transporters using real time RT-PCR. Free (64)Cu uptake in rat choroid plexus (CP), where the blood-cerebrospinal fluid barrier (BCB) is primarily located, is about 50 and 1000 times higher than (64)Cu-albumin and (64)Cu-ceruloplasmin uptake, respectively. The unidirectional transport rate constants (K(in)) for Cu in the CP and brain capillaries of the blood-brain barrier (BBB) were 1034 and 319 microl/s/g, respectively, while K(in) in CSF and capillary-depleted parenchyma were much reduced, 0.8 and 112 microl/s/g, respectively. The K(in) in cerebellum was significantly lower than that in hippocampus. The mRNAs encoding Cu transporter-1 (Ctr1) and ATP7A were higher in the CP than those in brain capillaries and parenchyma, whereas ATP7B mRNA was higher in brain capillaries than those in the CP and brain parenchyma. Taken together, these data suggest that the expression of Cu transporters is higher in brain barriers than in brain parenchyma; the Cu transport into the brain is mainly achieved through the BBB as a free Cu ion and the BCB may serve as a main regulatory site of Cu in the CSF.
Figures


References
-
- Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, Ross BM, Mekios C, Scheinberg IH, Gilliam TC. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum Mol Genet. 1999;8:1665–1671. - PubMed
-
- Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66:1198–1207. - PubMed
-
- Campbell CH, Brown R, Linder MC. Circulating ceruloplasmin is an important source of copper for normal and malignant animal cells. Biochim Biophys Acta. 1981;678:27–38. - PubMed
-
- Darwish HM, Cheney JC, Schmitt RC, Ettinger MJ. Mobilization of copper(II) from plasma components and mechanisms of hepatic copper transport. Am J Physiol. 1984;246:G72–G79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

