Contributions of the two accessory subunits, RNASEH2B and RNASEH2C, to the activity and properties of the human RNase H2 complex
- PMID: 19015152
- PMCID: PMC2615623
- DOI: 10.1093/nar/gkn913
Contributions of the two accessory subunits, RNASEH2B and RNASEH2C, to the activity and properties of the human RNase H2 complex
Abstract
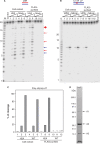
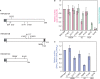
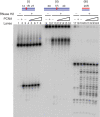
Eukaryotic RNase H2 is a heterotrimeric enzyme. Here, we show that the biochemical composition and stoichiometry of the human RNase H2 complex is consistent with the properties previously deduced from genetic studies. The catalytic subunit of eukaryotic RNase H2, RNASEH2A, is well conserved and similar to the monomeric prokaryotic RNase HII. In contrast, the RNASEH2B and RNASEH2C subunits from human and Saccharomyces cerevisiae share very little homology, although they both form soluble B/C complexes that may serve as a nucleation site for the addition of RNASEH2A to form an active RNase H2, or for interactions with other proteins to support different functions. The RNASEH2B subunit has a PIP-box and confers PCNA binding to human RNase H2. Unlike Escherichia coli RNase HII, eukaryotic RNase H2 acts processively and hydrolyzes a variety of RNA/DNA hybrids with similar efficiencies, suggesting multiple cellular substrates. Moreover, of five analyzed mutations in human RNASEH2B and RNASEH2C linked to Aicardi-Goutières Syndrome (AGS), only one, R69W in the RNASEH2C protein, exhibits a significant reduction in specific activity, revealing a role for the C subunit in enzymatic activity. Near-normal activity of four AGS-related mutant enzymes was unexpected in light of their predicted impairment causing the AGS phenotype.
Figures





References
-
- Crouch RJ, Toulme JJ, editors. Ribonucleases H. Paris: INSERM; 1998.
-
- Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, Ali M, Semple C, Aicardi J, Babul-Hirji R, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutières syndrome and mimic congenital viral brain infection. Nat. Genet. 2006;38:910–916. - PubMed
-
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, et al. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutières syndrome at the AGS1 locus. Nat. Genet. 2006;38:917–920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

