Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study
- PMID: 19015206
- PMCID: PMC2674555
- DOI: 10.1136/ard.2008.099291
Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study
Abstract
Background: Tumour necrosis factor alpha (TNFalpha) is a proinflammatory cytokine involved in the pathogenesis of rheumatoid arthritis (RA). Treatment with TNFalpha inhibitors reduces disease activity and improves outcomes for patients with RA. This study evaluated the efficacy and safety of certolizumab pegol 400 mg, a novel, poly-(ethylene glycol) (PEG)ylated, Fc-free TNFalpha inhibitor, as monotherapy in patients with active RA.
Methods: In this 24-week, multicentre, randomised, double-blind, placebo-controlled study, 220 patients previously failing > or =1 disease-modifying antirheumatic drug (DMARD) were randomised 1:1 to receive subcutaneous certolizumab pegol 400 mg (n = 111) or placebo (n = 109) every 4 weeks. The primary endpoint was 20% improvement according to the American College of Rheumatology criteria (ACR20) at week 24. Secondary endpoints included ACR50/70 response, ACR component scores, 28-joint Disease Activity Score Erythrocyte Sedimentation Rate 3 (DAS28(ESR)3), patient-reported outcomes (including physical function, health-related quality of life (HRQoL), pain and fatigue) and safety.
Results: At week 24, the ACR20 response rates were 45.5% for certolizumab pegol 400 mg every 4 weeks vs 9.3% for placebo (p<0.001). Differences for certolizumab pegol vs placebo in the ACR20 response were statistically significant as early as week 1 through to week 24 (p<0.001). Significant improvements in ACR50, ACR components, DAS28(ESR)3 and all patient-reported outcomes were also observed early with certolizumab pegol and were sustained throughout the study. Most adverse events were mild or moderate and no deaths or cases of tuberculosis were reported.
Conclusions: Treatment with certolizumab pegol 400 mg monotherapy every 4 weeks effectively reduced the signs and symptoms of active RA in patients previously failing > or =1 DMARD compared with placebo, and demonstrated an acceptable safety profile.
Trial registration number: NCT00548834.
Conflict of interest statement
Figures

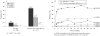

Comment in
-
New tumour necrosis factor inhibitors for rheumatoid arthritis: are there benefits from extending choice?Ann Rheum Dis. 2009 Jun;68(6):767-9. doi: 10.1136/ard.2008.105940. Ann Rheum Dis. 2009. PMID: 19435722 No abstract available.
References
-
- Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9 - PubMed
-
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, et al. Etanercept therapy in rheumatoid arthritis: a randomized, controlled trial. Ann Intern Med 1999;130:478–86 - PubMed
-
- Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9 - PubMed
-
- Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum 2004;50:1400–11 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

