Artemis and nonhomologous end joining-independent influence of DNA-dependent protein kinase catalytic subunit on chromosome stability
- PMID: 19015239
- PMCID: PMC2612508
- DOI: 10.1128/MCB.01354-08
Artemis and nonhomologous end joining-independent influence of DNA-dependent protein kinase catalytic subunit on chromosome stability
Abstract
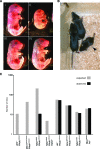
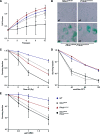
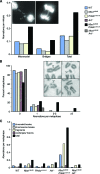
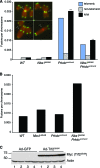
Deficiency in both ATM and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is synthetically lethal in developing mouse embryos. Using mice that phenocopy diverse aspects of Atm deficiency, we have analyzed the genetic requirements for embryonic lethality in the absence of functional DNA-PKcs. Similar to the loss of ATM, hypomorphic mutations of Mre11 (Mre11(ATLD1)) led to synthetic lethality when juxtaposed with DNA-PKcs deficiency (Prkdc(scid)). In contrast, the more moderate DNA double-strand break response defects associated with the Nbs1(DeltaB) allele permitted viability of some Nbs1(DeltaB/DeltaB) Prkdc(scid/scid) embryos. Cell cultures from Nbs1(DeltaB/DeltaB) Prkdc(scid/scid) embryos displayed severe defects, including premature senescence, mitotic aberrations, sensitivity to ionizing radiation, altered checkpoint responses, and increased chromosome instability. The known functions of DNA-PKcs in the regulation of Artemis nuclease activity or nonhomologous end joining-mediated repair do not appear to underlie the severe genetic interaction. Our results reveal a role for DNA-PKcs in the maintenance of S/G(2)-phase chromosome stability and in the induction of cell cycle checkpoint responses.
Figures
References
-
- Bailey, S. M., M. N. Cornforth, A. Kurimasa, D. J. Chen, and E. H. Goodwin. 2001. Strand-specific postreplicative processing of mammalian telomeres. Science 2932462-2465. - PubMed
-
- Carney, J. P., R. S. Maser, H. Olivares, E. M. Davis, M. Le Beau, J. R. Yates III, L. Hays, W. F. Morgan, and J. H. Petrini. 1998. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell 93477-486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
