Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice
- PMID: 19015248
- PMCID: PMC2632015
- DOI: 10.1128/IAI.00994-08
Dendritic cell and NK cell reciprocal cross talk promotes gamma interferon-dependent immunity to blood-stage Plasmodium chabaudi AS infection in mice
Abstract
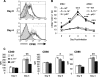
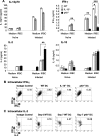
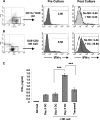
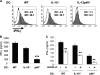
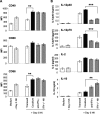
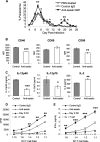
Dendritic cells (DCs) are important accessory cells for promoting NK cell gamma interferon (IFN-gamma) production in vitro in response to Plasmodium falciparum-infected red blood cells (iRBC). We investigated the requirements for reciprocal activation of DCs and NK cells leading to Th1-type innate and adaptive immunity to P. chabaudi AS infection. During the first week of infection, the uptake of iRBC by splenic CD11c(+) DCs in resistant wild-type (WT) C57BL/6 mice was similar to that in interleukin 15(-/-) (IL-15(-/-)) and IL-12p40(-/-) mice, which differ in the severity of P. chabaudi AS infection. DCs from infected IL-15(-/-) mice expressed costimulatory molecules, produced IL-12, and promoted IFN-gamma secretion by WT NK cells in vitro as efficiently as WT DCs. In contrast, DCs from infected IL-12p40(-/-) mice exhibited alterations in maturation and cytokine production and were unable to induce NK cell IFN-gamma production. Coculture of DCs and NK cells demonstrated that DC-mediated NK cell activation required IL-12 and, to a lesser extent, IL-2, as well as cell-cell contact. In turn, NK cells from infected WT mice enhanced DC maturation, IL-12 production, and priming of CD4(+) T-cell proliferation and IFN-gamma secretion. Infected WT mice depleted of NK cells, which exhibit increased parasitemia, had impaired DC maturation and DC-induced CD4(+) Th1 cell priming. These findings indicate that DC-NK cell reciprocal cross talk is critical for control and rapid resolution of P. chabaudi AS infection and provide in vivo evidence for the importance of this interaction in IFN-gamma-dependent immunity to malaria.
Figures

References
-
- Ahvazi, B. C., P. Jacobs, and M. M. Stevenson. 1995. Role of macrophage-derived nitric oxide in suppression of lymphocyte proliferation during blood-stage malaria. J. Leukoc. Biol. 5823-31. - PubMed
-
- Alli, R. S., and A. Khar. 2004. Interleukin-12 secreted by mature dendritic cells mediates activation of NK cell function. FEBS Lett. 55971-76. - PubMed
-
- Artavanis-Tsakonas, K., K. Eleme, K. L. McQueen, N. W. Cheng, P. Parham, D. M. Davis, and E. M. Riley. 2003. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J. Immunol. 1715396-5405. - PubMed
-
- Artavanis-Tsakonas, K., and E. M. Riley. 2002. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 1692956-2963. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18767-811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

