Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction
- PMID: 19015269
- PMCID: PMC2615530
- DOI: 10.1074/jbc.M802298200
Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction
Abstract
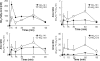
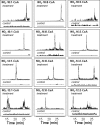
Nitrated derivatives of fatty acids (NO2-FA) are pluripotent cell-signaling mediators that display anti-inflammatory properties. Current understanding of NO2-FA signal transduction lacks insight into how or if NO2-FA are modified or metabolized upon formation or administration in vivo. Here the disposition and metabolism of nitro-9-cis-octadecenoic (18:1-NO2) acid was investigated in plasma and liver after intravenous injection in mice. High performance liquid chromatography-tandem mass spectrometry analysis showed that no 18:1-NO2 or metabolites were detected under basal conditions, whereas administered 18:1-NO2 is rapidly adducted to plasma thiol-containing proteins and glutathione. NO2-FA are also metabolized via beta-oxidation, with high performance liquid chromatography-tandem mass spectrometry analysis of liver lipid extracts of treated mice revealing nitro-7-cis-hexadecenoic acid, nitro-5-cis-tetradecenoic acid, and nitro-3-cis-dodecenoic acid and corresponding coenzyme A derivatives of 18:1-NO2 as metabolites. Additionally, a significant proportion of 18:1-NO2 and its metabolites are converted to nitroalkane derivatives by saturation of the double bond, and to a lesser extent are desaturated to diene derivatives. There was no evidence of the formation of nitrohydroxyl or conjugated ketone derivatives in organs of interest, metabolites expected upon 18:1-NO2 hydration or nitric oxide (*NO) release. Plasma samples from treated mice had significant extents of protein-adducted 18:1-NO2 detected by exchange to added beta-mercaptoethanol. This, coupled with the observation of 18:1-NO2 release from glutathione-18:1-NO2 adducts, supports that reversible and exchangeable NO2-FA-thiol adducts occur under biological conditions. After administration of [3H]18:1-NO2, 64% of net radiolabel was recovered 90 min later in plasma (0.2%), liver (18%), kidney (2%), adipose tissue (2%), muscle (31%), urine (6%), and other tissue compartments, and may include metabolites not yet identified. In aggregate, these findings show that electrophilic FA nitroalkene derivatives (a) acquire an extended half-life by undergoing reversible and exchangeable electrophilic reactions with nucleophilic targets and (b) are metabolized predominantly via saturation of the double bond and beta-oxidation reactions that terminate at the site of acyl-chain nitration.
Figures





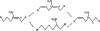





References
-
- O'Donnell, V. B., Eiserich, J. P., Chumley, P. H., Jablonsky, M. J., Krishna, N. R., Kirk, M., Barnes, S., rley-Usmar, V. M., and Freeman, B. A. (1999) Chem. Res. Toxicol. 12 83–92 - PubMed
-
- Napolitano, A., Camera, E., Picardo, M., and d'Ischia, M. (2000) J. Org. Chem. 65 4853–4860 - PubMed
-
- Lima, E. S., Di, M. P., and Abdalla, D. S. (2003) J. Lipid Res. 44 1660–1666 - PubMed
-
- Finlayson-Pitts, B. J., Sweetman, L. L., and Weissbart, B. (1987) Toxicol. Appl. Pharmacol. 89 438–448 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

