A point mutation in the murine Hem1 gene reveals an essential role for Hematopoietic protein 1 in lymphopoiesis and innate immunity
- PMID: 19015308
- PMCID: PMC2585840
- DOI: 10.1084/jem.20080340
A point mutation in the murine Hem1 gene reveals an essential role for Hematopoietic protein 1 in lymphopoiesis and innate immunity
Abstract
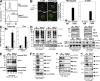
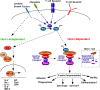
Hem1 (Hematopoietic protein 1) is a hematopoietic cell-specific member of the Hem family of cytoplasmic adaptor proteins. Orthologues of Hem1 in Dictyostelium discoideum, Drosophila melanogaster, and Caenorhabditis elegans are essential for cytoskeletal reorganization, embryonic cell migration, and morphogenesis. However, the in vivo functions of mammalian Hem1 are not known. Using a chemical mutagenesis strategy in mice to identify novel genes involved in immune cell functions, we positionally cloned a nonsense mutation in the Hem1 gene. Hem1 deficiency results in defective F-actin polymerization and actin capping in lymphocytes and neutrophils caused by loss of the Rac-controlled actin-regulatory WAVE protein complex. T cell development is disrupted in Hem1-deficient mice at the CD4(-)CD8(-) (double negative) to CD4(+)CD8(+) (double positive) cell stages, whereas T cell activation and adhesion are impaired. Hem1-deficient neutrophils fail to migrate in response to chemotactic agents and are deficient in their ability to phagocytose bacteria. Remarkably, some Rac-dependent functions, such as Th1 differentiation and nuclear factor kappaB (NF-kappaB)-dependent transcription of proinflammatory cytokines proceed normally in Hem1-deficient mice, whereas the production of Th17 cells are enhanced. These results demonstrate that Hem1 is essential for hematopoietic cell development, function, and homeostasis by controlling a distinct pathway leading to cytoskeletal reorganization, whereas NF-kappaB-dependent transcription proceeds independently of Hem1 and F-actin polymerization.
Figures






References
-
- Weston, V.J., and T. Stankovic. 2004. Rac1 and Rac2 GTPases in haematopoiesis. Bioessays. 26:221–224. - PubMed
-
- Li, B., H. Yu, W. Zheng, R. Voll, S. Na, A.W. Roberts, D.A. Williams, R.J. Davis, S. Ghosh, and R.A. Flavell. 2000. Role of the guanosine triphosphatase Rac2 in T helper 1 cell differentiation. Science. 288:2219–2222. - PubMed
-
- Benvenuti, F., S. Hugues, M. Walmsley, S. Ruf, L. Fetler, M. Popoff, V.L. Tybulewicz, and S. Amigorena. 2004. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 305:1150–1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

