HP1-beta is required for development of the cerebral neocortex and neuromuscular junctions
- PMID: 19015315
- PMCID: PMC2582898
- DOI: 10.1083/jcb.200804041
HP1-beta is required for development of the cerebral neocortex and neuromuscular junctions
Abstract
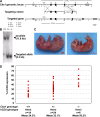
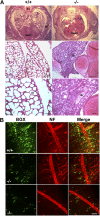
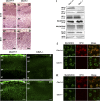
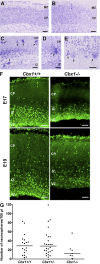
HP1 proteins are thought to be modulators of chromatin organization in all mammals, yet their exact physiological function remains unknown. In a first attempt to elucidate the function of these proteins in vivo, we disrupted the murine Cbx1 gene, which encodes the HP1-beta isotype, and show that the Cbx1(-/-) -null mutation leads to perinatal lethality. The newborn mice succumbed to acute respiratory failure, whose likely cause is the defective development of neuromuscular junctions within the endplate of the diaphragm. We also observe aberrant cerebral cortex development in Cbx1(-/-) mutant brains, which have reduced proliferation of neuronal precursors, widespread cell death, and edema. In vitro cultures of neurospheres from Cbx1(-/-) mutant brains reveal a dramatic genomic instability. Our results demonstrate that HP1 proteins are not functionally redundant and that they are likely to regulate lineage-specific changes in heterochromatin organization.
Figures
References
-
- Cheutin, T., A.J. McNairn, T. Jenuwein, D.M. Gilbert, P.B. Singh, and T. Misteli. 2003. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 299:721–725. - PubMed
-
- Collombet, J.M., C. Masqueliez, E. Four, M.F. Burckhart, D. Bernabé, D. Baubichon, and G. Lallement. 2006. Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci. Lett. 398:337–342. - PubMed
-
- Cordelires, F., and J. Jackson. 2007. 3D Object Counter. National Institutes of Health, Baltimore, MD. http://rsb.info.nih.gov/ij/plugins/track/objects.html (accessed August, 2008).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

