Inside information: the unique features of visceral sensation
- PMID: 19015388
- PMCID: PMC2732716
- DOI: 10.1124/mi.8.5.9
Inside information: the unique features of visceral sensation
Abstract
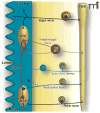
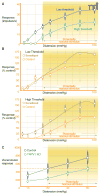
Most of what is written and believed about pain and nociceptors originates from studies of the "somatic" (non-visceral) sensory system. As a result, the unique features of visceral pain are often overlooked. In the clinic, the management of visceral pain is typically poor, and drugs that are used with some efficacy to treat somatic pain often present unwanted effects on the viscera. For these reasons, a better understanding of visceral sensory neurons-particularly visceral nociceptors-is required. This review provides evidence of functional, morphological, and biochemical differences between visceral and non-visceral afferents, with a focus on potential nociceptive roles, and also considers some of the potential mechanisms of visceral mechanosensation.
Figures

References
-
- Grundy D. Signalling the state of the digestive tract. Auton Neurosci. 2006;125:76–80. - PubMed
-
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19(Suppl 1):1–19. - PubMed
-
- Cervero F. Sensory innervation of the viscera: Peripheral basis of visceral pain. Physiol Rev. 1994;74:95–138. - PubMed
-
- Sengupta JN, Gebhart GF. Gastrointestinal afferent fibers and sensation. In: Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. Raven Press; New York: 1994. pp. 483–519.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
