Electrophysiological consequences of acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers
- PMID: 19015404
- PMCID: PMC2730415
- DOI: 10.1161/CIRCULATIONAHA.108.789149
Electrophysiological consequences of acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers
Abstract
Background: Electrophysiological changes promoting arrhythmias during acute regional ischemia/reperfusion are challenging to study in intact cardiac tissue because of complex 3-dimensional myocardial and vascular geometry. We characterized electrophysiological alterations and arrhythmias during regional ischemia/reperfusion in a simpler 2-dimensional geometry of cultured neonatal rat ventricular myocyte monolayers.
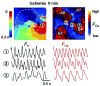
Methods and results: Optical mapping of intracellular Ca (Ca(i)) and voltage was performed with the use of Rhod 2-AM and Rh-237, respectively. Regional ischemia was mimicked by covering the central portion of monolayer with a glass coverslip, and reperfusion was mimicked by removing the coverslip. Monolayers were stained with fluorescent antibodies to detect total and dephosphorylated connexin-43 at various time points. During coverslip ischemia, action potential duration shortened, Ca(i) transient duration was prolonged, and local conduction velocity (CV) slowed progressively, with loss of excitability after 10.6 +/- 3.6 minutes. CV slowing was accompanied by connexin-43 dephosphorylation. During ischemia, spontaneous reentry occurred in 5 of 11 monolayers, initiated by extrasystoles arising from the border zone or unidirectional conduction block of paced beats. On reperfusion, excitability recovered within 1.0 +/- 0.8 minutes, but CV remained depressed for 9.0 +/- 3.0 minutes, promoting reentry in the reperfused zone. As connexin-43 phosphorylation recovered in the reperfused zone, CV normalized, and arrhythmias resolved.
Conclusions: Acute regional ischemia/reperfusion in neonatal rat ventricular myocyte monolayers recapitulates electrophysiological alterations and arrhythmias similar to those observed during acute coronary occlusion/reperfusion in intact hearts. During early reperfusion, slow recovery from connexin-43 dephosphorylation leads to persistent CV slowing, creating a highly arrhythmogenic substrate.
Conflict of interest statement
Figures



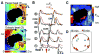


Similar articles
-
Mitochondrial instability during regional ischemia-reperfusion underlies arrhythmias in monolayers of cardiomyocytes.J Mol Cell Cardiol. 2015 Jan;78:90-9. doi: 10.1016/j.yjmcc.2014.09.024. Epub 2014 Sep 28. J Mol Cell Cardiol. 2015. PMID: 25268650 Free PMC article.
-
Nutrient restriction preserves calcium cycling and mitochondrial function in cardiac myocytes during ischemia and reperfusion.Cell Calcium. 2012 Jun;51(6):445-51. doi: 10.1016/j.ceca.2012.02.009. Epub 2012 Mar 17. Cell Calcium. 2012. PMID: 22424693 Free PMC article.
-
Connexin 43 dephosphorylation contributes to arrhythmias and cardiomyocyte apoptosis in ischemia/reperfusion hearts.Basic Res Cardiol. 2019 Aug 28;114(5):40. doi: 10.1007/s00395-019-0748-8. Basic Res Cardiol. 2019. PMID: 31463533
-
Mechanisms of ventricular arrhythmias in acute ischemia and reperfusion.Cardiovasc Clin. 1992;22(1):3-18. Cardiovasc Clin. 1992. PMID: 1728431 Review.
-
Optical imaging of arrhythmias in the cardiomyocyte monolayer.Heart Rhythm. 2012 Dec;9(12):2077-82. doi: 10.1016/j.hrthm.2012.08.035. Epub 2012 Aug 29. Heart Rhythm. 2012. PMID: 23108055 Review.
Cited by
-
Taxol, a microtubule stabilizer, prevents ischemic ventricular arrhythmias in rats.J Cell Mol Med. 2011 May;15(5):1166-76. doi: 10.1111/j.1582-4934.2010.01106.x. Epub 2010 Jun 17. J Cell Mol Med. 2011. PMID: 20561109 Free PMC article.
-
Stem cells can form gap junctions with cardiac myocytes and exert pro-arrhythmic effects.Front Physiol. 2014 Oct 29;5:419. doi: 10.3389/fphys.2014.00419. eCollection 2014. Front Physiol. 2014. PMID: 25400586 Free PMC article. Review.
-
LRP6-mediated phosphorylation of connexin43 in myocardial infarction.iScience. 2023 Feb 8;26(3):106160. doi: 10.1016/j.isci.2023.106160. eCollection 2023 Mar 17. iScience. 2023. PMID: 36879803 Free PMC article.
-
Ratiometric imaging of calcium during ischemia-reperfusion injury in isolated mouse hearts using Fura-2.Biomed Eng Online. 2012 Jul 19;11:39. doi: 10.1186/1475-925X-11-39. Biomed Eng Online. 2012. PMID: 22812644 Free PMC article.
-
β2-Adrenergic Receptor Agonist Clenbuterol Protects Against Acute Ischemia/Reperfusion-Induced Arrhythmia by Regulation of Akt/eNOS/NO/Cx43 Signaling Pathway.Pharmacol Res Perspect. 2025 Feb;13(1):e70070. doi: 10.1002/prp2.70070. Pharmacol Res Perspect. 2025. PMID: 39873977 Free PMC article.
References
-
- Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. - PubMed
-
- Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. - PubMed
-
- Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69:1049–1169. - PubMed
-
- Kleber AG, Riegger CB, Janse MJ. Electrical uncoupling and increase of extracellular resistance after induction of ischemia in isolated, arterially perfused rabbit papillary muscle. Circ Res. 1987;61:271–279. - PubMed
-
- Weiss J, Shine KI. [K+]o accumulation and electrophysiological alterations during early myocardial ischemia. Am J Physiol. 1982;243:318–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

