IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells
- PMID: 19015529
- PMCID: PMC2587589
- DOI: 10.1073/pnas.0809850105
IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells
Abstract
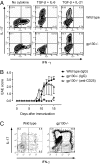
The conditions leading to the induction of adaptive Foxp3(+) regulatory T cells (T-regs) from peripheral T cells in vivo are incompletely understood. Here, we show that unresponsiveness of T cells to IL-6 by T cell-selective deletion of gp130 or immunization of wild-type mice with antigen in incomplete Freund's adjuvant (IFA), which fails to induce IL-6, promotes the conversion of peripheral CD4(+) T cells into adaptive Foxp3(+) T-regs. Thus, both T cell-conditional gp130 knockout (KO) mice immunized with MOG35-55 in complete Freund's adjuvant (CFA) and wild-type mice immunized with MOG35-55 in IFA develop overwhelming antigen-specific T-reg responses and are protected from experimental autoimmune encephalomyelitis (EAE). Depletion of T-regs restores T helper (Th)17 responses and clinical EAE in MOG/CFA-immunized T cell-conditional gp130 KO mice, but not in MOG/IFA-immunized wild-type mice. We conclude that in the absence of T-regs, IL-6 signaling is dispensable for the induction of Th17 cells, and alternative pathways exist to induce Th17 cells and EAE in the absence of IL-6 signaling. However, IL-6 signaling is dominant in inhibiting the conversion of conventional T cells into Foxp3(+) T-regs in vivo, and in the absence of IL-6 signaling, no other cytokine can substitute in inhibiting T-reg conversion. These data identify IL-6 as an important target to modulate autoimmune responses and chronic inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. - PubMed
-
- Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. - PubMed
-
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. - PubMed
-
- Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1P01NS38037-04/NS/NINDS NIH HHS/United States
- 2R01NS35685-06/NS/NINDS NIH HHS/United States
- R01 NS045937/NS/NINDS NIH HHS/United States
- R01 NS035685/NS/NINDS NIH HHS/United States
- R01AI073542-01/AI/NIAID NIH HHS/United States
- 2R37NS30843-11/NS/NINDS NIH HHS/United States
- 1R01A144880-03/PHS HHS/United States
- R01 AI073542/AI/NIAID NIH HHS/United States
- R37 NS030843/NS/NINDS NIH HHS/United States
- 2P01A139671-07/PHS HHS/United States
- 1R01NS045937-01/NS/NINDS NIH HHS/United States
- R01 NS046414/NS/NINDS NIH HHS/United States
- 1R01NS046414/NS/NINDS NIH HHS/United States
- P01 NS038037/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

