The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era
- PMID: 19016329
- PMCID: PMC2682636
- DOI: 10.1007/s11481-008-9136-0
The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era
Abstract
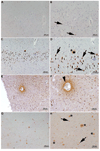
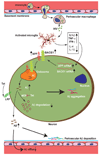
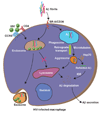
Although the introduction of highly active antiretroviral therapy (HAART) has led to a strong reduction of HIV-associated dementia (HAD) incidence, the prevalence of minor HIV-1-associated neurocognitive disorder (HAND) is rising among AIDS patients. HAART medication has shifted neuropathology from a subacute encephalitic condition to a subtle neurodegenerative process involving synaptic and dendritic degeneration, particularly of hippocampal neurons that are spared prior to HAART medication. Considerable neuroinflammation coupled with mononuclear phagocyte activation is present in HAART-medicated brains, particularly in the hippocampus. Accumulating evidence suggests that the resultant elevated secretion of pro-inflammatory cytokines such as interferon-gamma, tumor necrosis factor-alpha, and interleukin-1beta can increase amyloid-beta peptide (Abeta) generation and reduce Abeta clearance. Recent advancements in Alzheimer's disease (AD) research identified Abeta biogenesis and clearance venues that are potentially influenced by HIV viral infection, providing new insights into beta-amyloidosis segregation in HIV patients. Our study suggests enhanced beta-amyloidosis in ART-treated HAD and HIV-associated encephalitis brains and suppression of Abeta clearance by viral infection of human primary macrophages. A growing awareness of potential convergent mechanisms leading to neurodegeneration shared by HIV and Abeta points to a significant chance of comorbidity of AD and HAND in senile HIV patients, which calls for a need of basic studies.
Figures

References
-
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. - PubMed
-
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. - PubMed
-
- An SF, Groves M, Gray F, Scaravilli F. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. - PubMed
-
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. - PubMed
-
- Andersson L, Blennow K, Fuchs D, Svennerholm B, Gisslen M. Increased cerebrospinal fluid protein tau concentration in neuro-AIDS. J Neurol Sci. 1999;171:92–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

