Expression of interferon-gamma and tumour necrosis factor-alpha messenger RNA does not correlate with protection in guinea pigs challenged with virulent Mycobacterium tuberculosis by the respiratory route
- PMID: 19016908
- PMCID: PMC2753903
- DOI: 10.1111/j.1365-2567.2008.02962.x
Expression of interferon-gamma and tumour necrosis factor-alpha messenger RNA does not correlate with protection in guinea pigs challenged with virulent Mycobacterium tuberculosis by the respiratory route
Abstract
Cytokine messenger RNA (mRNA) expression was investigated in the spleen and lung digest cells of bacillus Calmette-Guérin (BCG)-vaccinated and non-vaccinated guinea pigs following low-dose, pulmonary exposure to virulent Mycobacterium tuberculosis. After purified protein derivative (PPD) stimulation, the levels of lung cell interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha) and spleen cell interleukin-12 (IL-12) p40 mRNAs were significantly increased in the non-vaccinated M. tuberculosis-infected guinea pigs compared to the BCG-vaccinated guinea pigs. In contrast, the expression of anti-inflammatory transforming growth factor-beta and IL-10 mRNAs was significantly enhanced in the spleens of BCG-vaccinated animals. Despite the presence of protective cytokine mRNA expression, the non-vaccinated guinea pigs had significantly higher lung and spleen bacterial burdens. In contrast, BCG-vaccinated guinea pigs controlled the bacterial multiplication in their lungs and spleens, indicating that both protective as well as anti-inflammatory cytokine responses are associated with a reduction in bacteria. In addition, lung digest cells from non-vaccinated guinea pigs contained a significantly higher percentage of neutrophils, CD3(+) and CD8(+) T cells, while the percentage of macrophages was increased in the BCG-vaccinated animals. Total and purified lung digest T cells co-cultured with lung macrophages (LMøs) proliferated poorly after PPD stimulation in both non-vaccinated and BCG-vaccinated animals while robust proliferation to PPD was observed when T cells were co-cultured with peritoneal macrophages (PMøs). Macrophages within the lung compartment appear to regulate the response of T cells irrespective of the vaccination status in guinea pigs. Taken together, our results suggest that type I cytokine mRNA expression is not associated with vaccine-induced protection in the low-dose guinea pig model of tuberculosis.
Figures
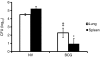
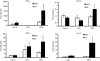

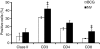
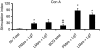
References
-
- Kaufmann S. Towards new leprosy and tuberculosis vaccines. Microbiol Sci. 1987;4:324–8. - PubMed
-
- Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. - PubMed
-
- Young LS, Inderlied CB, Berlin OG, Gottlieb MS. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986;8:1024–33. - PubMed
-
- Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. - PubMed
-
- Smith D, Harding G, Chan J, Edwards M, Hank J, Muller D, Sobhi F. Potency of 10 BCG vaccines as evaluated by their influence on the bacillemic phase of experimental airborne tuberculosis in guinea pigs. J Biol Stand. 1979;7:179–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

