The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair
- PMID: 19017790
- PMCID: PMC2587631
- DOI: 10.1073/pnas.0803437105
The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair
Abstract
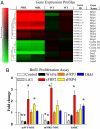
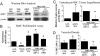
Cell-based therapies, using multipotent mesenchymal stem cells (MSCs) for organ regeneration, are being pursued for cardiac disease, orthopedic injuries and biomaterial fabrication. The molecular pathways that regulate MSC-mediated regeneration or enhance their therapeutic efficacy are, however, poorly understood. We compared MSCs isolated from MRL/MpJ mice, known to demonstrate enhanced regenerative capacity, to those from C57BL/6 (WT) mice. Compared with WT-MSCs, MRL-MSCs demonstrated increased proliferation, in vivo engraftment, experimental granulation tissue reconstitution, and tissue vascularity in a murine model of repair stimulation. The MRL-MSCs also reduced infarct size and improved function in a murine myocardial infarct model compared with WT-MSCs. Genomic and functional analysis indicated a downregulation of the canonical Wnt pathway in MRL-MSCs characterized by significant up-regulation of specific secreted frizzled-related proteins (sFRPs). Specific knockdown of sFRP2 by shRNA in MRL-MSCs decreased their proliferation and their engraftment in and the vascular density of MRL-MSC-generated experimental granulation tissue. These results led us to generate WT-MSCs overexpressing sFRP2 (sFRP2-MSCs) by retroviral transduction. sFRP2-MSCs maintained their ability for multilineage differentiation in vitro and, when implanted in vivo, recapitulated the MRL phenotype. Peri-infarct intramyocardial injection of sFRP2-MSCs resulted in enhanced engraftment, vascular density, reduced infarct size, and increased cardiac function after myocardial injury in mice. These findings implicate sFRP2 as a key molecule for the biogenesis of a superior regenerative phenotype in MSCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
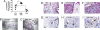


References
-
- Kinnaird T, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circ. 2004;109:1543–1549. - PubMed
-
- Falanga V, et al. Autologous BM-derived cultured MSCs delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: Mesenchymal stem/multipotent stromal cells: The state of transdifferentiation and models of tissue repair-current views. Stem Cells. 2007;25:2896–2902. - PubMed
-
- Jiang Y, et al. Pluripotency of MSCs derived from adult marrow. Nature. 2002;418:41–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

