Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome
- PMID: 19017793
- PMCID: PMC2587583
- DOI: 10.1073/pnas.0807967105
Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome
Abstract
The underlying mechanism by which anti-VEGF agents prolong cancer patient survival is poorly understood. We show that in a mouse tumor model, VEGF systemically impairs functions of multiple organs including those in the hematopoietic and endocrine systems, leading to early death. Anti-VEGF antibody, bevacizumab, and anti-VEGF receptor 2 (VEGFR-2), but not anti-VEGFR-1, reversed VEGF-induced cancer-associated systemic syndrome (CASS) and prevented death in tumor-bearing mice. Surprisingly, VEGFR2 blockage improved survival by rescuing mice from CASS without significantly compromising tumor growth, suggesting that "off-tumor" VEGF targets are more sensitive than the tumor vasculature to anti-VEGF drugs. Similarly, VEGF-induced CASS occurred in a spontaneous breast cancer mouse model overexpressing neu. Clinically, VEGF expression and CASS severity positively correlated in various human cancers. These findings define novel therapeutic targets of anti-VEGF agents and provide mechanistic insights into the action of this new class of clinically available anti-VEGF cancer drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
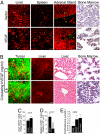

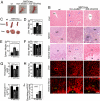
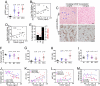
References
-
- Hurwitz H. Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin Colorectal Cancer. 2004;4(Suppl 2):S62–S68. - PubMed
-
- Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. - PubMed
-
- Ledford H. Drug markers questioned. Nature. 2008;452:510–511. - PubMed
-
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. - PubMed
-
- Nathanson L, Hall TC. Introduction: Paraneoplastic syndromes. Semin Oncol. 1997;24:265–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

