An endothelin-1 switch specifies maxillomandibular identity
- PMID: 19017795
- PMCID: PMC2596216
- DOI: 10.1073/pnas.0807345105
An endothelin-1 switch specifies maxillomandibular identity
Abstract
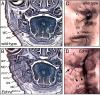
Articulated jaws are highly conserved structures characteristic of gnathostome evolution. Epithelial-mesenchymal interactions within the first pharyngeal arch (PA1) instruct cephalic neural crest cells (CNCCs) to form the different skeletal elements of the jaws. The endothelin-1 (Edn1)/endothelin receptor type-A (Ednra)-->Dlx5/6-->Hand2 signaling pathway is necessary for lower jaw formation. Here, we show that the Edn1 signaling is sufficient for the conversion of the maxillary arch to mandibular identity. Constitutive activation of Ednra induced the transformation of upper jaw, maxillary, structures into lower jaw, mandibular, structures with duplicated Meckel's cartilage and dermatocranial jaws constituted by 4 dentary bones. Misexpression of Hand2 in the Ednra domain caused a similar transformation. Skeletal transformations are accompanied by neuromuscular remodeling. Ednra is expressed by most CNCCs, but its constitutive activation affects predominantly PA1. We conclude that after migration CNCCs are not all equivalent, suggesting that their specification occurs in sequential steps. Also, we show that, within PA1, CNCCs are competent to form both mandibular and maxillary structures and that an Edn1 switch is responsible for the choice of either morphogenetic program.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
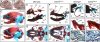



References
-
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development. 1993;117:409–429. - PubMed
-
- Creuzet S, Couly G, Vincent C, Le Douarin NM. Negative effect of Hox gene expression on the development of the neural crest-derived facial skeleton. Development. 2002;129:4301–4313. - PubMed
-
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. - PubMed
-
- Trainor PA, Tam PP. Cranial paraxial mesoderm and neural crest cells of the mouse embryo: Co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development. 1995;121:2569–2582. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

