Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-alpha/beta
- PMID: 19017955
- PMCID: PMC2676428
- DOI: 10.4049/jimmunol.181.11.7670
Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-alpha/beta
Abstract
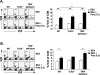
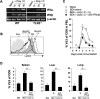
Poly(I:C) is an adjuvant used for antitumor treatment and vaccines because of its prominent effects on CD8 T cells and NK cells. Poly(I:C) binds TLR3 and this interaction is thought to be central for driving cell-mediated immune responses. We investigated the importance of TLR3 in poly(I:C)-mediated endogenous CD8 T cell responses using the pathogenic T cell stimulant Staphylococcus aureus enterotoxin A. While the responsive CD8 T cells expanded comparably in both wild-type and TLR3(-/-) mice, differentiation of effector CD8 T cells was enhanced by poly(I:C) in the TLR3(-/-) mice. A higher percentage of Ag-specific CD8 T cells became IFN-gamma and TNF-alpha producers in the absence of TLR3 signaling. Consistent with this boosted response was the observation that TLR3-deficient cells synthesized less IL-10 compared with TLR3-sufficient cells in response to poly(I:C). Ultimately, however, the fundamental mechanism of CD8 effector T cell differentiation through the TLR3-independent pathway was shown to be completely IFN-alpha/beta-dependent. Administration of IFN-alpha/beta-neutralizing Abs abolished the poly(I:C) effects in TLR3(-/-) mice. These findings reveal specific roles of how dsRNA receptors shape CD8 T cell responses, which should be considered as poly(I:C) is authenticated as a therapeutic adjuvant used in vaccines.
Figures




References
-
- Verdijk RM, Mutis T, Esendam B, Kamp J, Melief CJ, Brand A, Goulmy E. Polyriboinosinic polyribocytidylic acid (poly(I:C)) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. - PubMed
-
- Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008;180:683–687. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
-
- Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

