TREM-2 mediated signaling induces antigen uptake and retention in mature myeloid dendritic cells
- PMID: 19017976
- PMCID: PMC2606976
- DOI: 10.4049/jimmunol.181.11.7863
TREM-2 mediated signaling induces antigen uptake and retention in mature myeloid dendritic cells
Retraction in
-
Retraction: TREM-2 mediated signaling induces antigen uptake and retention in mature myeloid dendritic cells.J Immunol. 2010 Jun 1;184(11):6557. doi: 10.4049/jimmunol.1090036. J Immunol. 2010. PMID: 20483797 Free PMC article. No abstract available.
Abstract
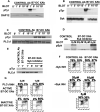
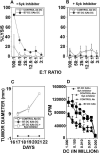
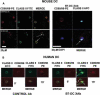
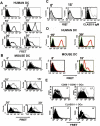
Myeloid dendritic cells (mDC) activated with a B7-DC-specific cross-linking IgM Ab (B7-DC XAb) take up and retain Ag and interact with T cell compartments to affect a number of biologic changes that together cause strong antitumor responses and blockade of inflammatory airway disease in animal models. The molecular events mediating the initial responses in mDC remain unclear. In this study we show that B7-DC XAb caused rapid phosphorylation of the adaptor protein DAP12 and intracellular kinases Syk and phospholipase C-gamma1. Pretreatment of mDC with the Syk inhibitor piceatannol blocked B7-DC XAb-induced Ag uptake with a concomitant loss of tumor protection in mice. Vaccination with tumor lysate-pulsed wild-type B7-DC XAb-activated mDC, but not TREM-2 knockout XAb-activated mDC, protected mice from lethal melanoma challenge. Multimolecular caps appeared within minutes of B7-DC XAb binding to either human or mouse mDC, and FRET analysis showed that class II, CD80, CD86, and TREM-2 are recruited in tight association on the cell surface. When TREM-2 expression was reduced in wild-type mDC using short hairpin RNA or by using mDC from TREM-2 knockout mice, in vitro DC failed to take up Ag after B7-DC XAb stimulation. These results directly link TREM-2 signaling with one change in the mDC phenotype that occurs in response to this unique Ab. The parallel signaling events observed in both human and mouse mDC support the hypothesis that B7-DC cross-linking may be useful as a therapeutic immune modulator in human patients.
Figures

References
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. - PubMed
-
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. - PubMed
-
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. - PubMed
-
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

