Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis
- PMID: 19017986
- PMCID: PMC2586986
- DOI: 10.4049/jimmunol.181.11.7948
Identification of human T cell antigens for the development of vaccines against Mycobacterium tuberculosis
Abstract
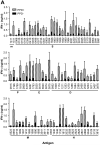
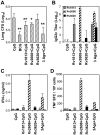
Development of a subunit vaccine for Mycobacterium tuberculosis (Mtb) depends on the identification of Ags that induce appropriate T cell responses. Using bioinformatics, we selected a panel of 94 Mtb genes based on criteria that included growth in macrophages, up- or down-regulation under hypoxic conditions, secretion, membrane association, or because they were members of the PE/PPE or EsX families. Recombinant proteins encoded by these genes were evaluated for IFN-gamma recall responses using PBMCs from healthy subjects previously exposed to Mtb. From this screen, dominant human T cell Ags were identified and 49 of these proteins, formulated in CpG, were evaluated as vaccine candidates in a mouse model of tuberculosis. Eighteen of the individual Ags conferred partial protection against challenge with virulent Mtb. A combination of three of these Ags further increased protection against Mtb to levels comparable to those achieved with bacillus Calmette-Guérin vaccination. Vaccine candidates that led to reduction in lung bacterial burden following challenge-induced pluripotent CD4 and CD8 T cells, including Th1 cell responses characterized by elevated levels of Ag-specific IgG2c, IFN-gamma, and TNF. Priority vaccine Ags elicited pluripotent CD4 and CD8 T responses in purified protein derivative-positive donor PBMCs. This study identified numerous novel human T cell Ags suitable to be included in subunit vaccines against tuberculosis.
Figures


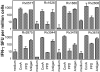

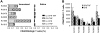


References
-
- WHO . Global Tuberculosis Control: Surveilance, Planning, Financing. World Health Organization; Geneva: 2007.
-
- Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007;370:2030–2043. - PubMed
-
- Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–662. - PubMed
-
- WHO . The WHO/IUTLD Global Project on Anti-Tuberculosis Resistance Surveillance. World Health Organization; Geneva: 2008. Anti-tuberculosis drug resistance in the world. Fourth global report.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

