Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells
- PMID: 19018007
- PMCID: PMC2586973
- DOI: 10.4049/jimmunol.181.11.8145
Differential expression and molecular associations of Syk in systemic lupus erythematosus T cells
Abstract
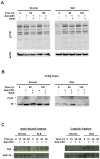
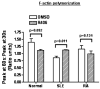
Diminished expression of TCR zeta and reciprocal up-regulation and association of FcRgamma with the TCR/CD3 complex is a hallmark of systemic lupus erythematosus (SLE) T cells. In this study we explored whether differential molecular associations of the spleen tyrosine kinase Syk that preferentially binds to FcRgamma contribute to pathological amplification of signals downstream of this "rewired TCR" in SLE. We detected higher amounts of Syk expression and activity in SLE compared with normal T cells. Selective inhibition of the activity of Syk reduced the strength of TCR-induced calcium responses and slowed the rapid kinetics of actin polymerization exclusively in SLE T cells. Syk and ZAP-70 also associated differently with key molecules involved in cytoskeletal and calcium signaling in SLE T cells. Thus, while Vav-1 and LAT preferentially bound to Syk, phospholipase C-gamma1 bound to both Syk and ZAP-70. Our results show that differential associations of Syk family kinases contribute to the enhanced TCR-induced signaling responses in SLE T cells. Thus, we propose molecular targeting of Syk as a measure to control abnormal T cell responses in SLE.
Figures



References
-
- Kammer GM, Perl A, Richardson BC, Tsokos GC. Abnormal T cell signal transduction in systemic lupus erythematosus. Arthritis Rheum. 2002;46:1139–1154. - PubMed
-
- Kong PL, Odegard JM, Bouzahzah F, Choi JY, Eardley LD, Zielinski CE, Craft JE. Intrinsic T cell defects in systemic autoimmunity. Ann NY Acad Sci. 2003;987:60–67. - PubMed
-
- Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, Tsokos GC. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172:7821–7831. - PubMed
-
- Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

