New paradigm for macromolecular crystallography experiments at SSRL: automated crystal screening and remote data collection
- PMID: 19018097
- PMCID: PMC2631117
- DOI: 10.1107/S0907444908030564
New paradigm for macromolecular crystallography experiments at SSRL: automated crystal screening and remote data collection
Abstract
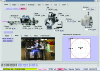
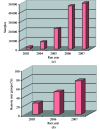
Complete automation of the macromolecular crystallography experiment has been achieved at SSRL through the combination of robust mechanized experimental hardware and a flexible control system with an intuitive user interface. These highly reliable systems have enabled crystallography experiments to be carried out from the researchers' home institutions and other remote locations while retaining complete control over even the most challenging systems. A breakthrough component of the system, the Stanford Auto-Mounter (SAM), has enabled the efficient mounting of cryocooled samples without human intervention. Taking advantage of this automation, researchers have successfully screened more than 200 000 samples to select the crystals with the best diffraction quality for data collection as well as to determine optimal crystallization and cryocooling conditions. These systems, which have been deployed on all SSRL macromolecular crystallography beamlines and several beamlines worldwide, are used by more than 80 research groups in remote locations, establishing a new paradigm for macromolecular crystallography experimentation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

