Economic evaluation of fulvestrant as an extra step in the treatment sequence for ER-positive advanced breast cancer
- PMID: 19018261
- PMCID: PMC2607221
- DOI: 10.1038/sj.bjc.6604790
Economic evaluation of fulvestrant as an extra step in the treatment sequence for ER-positive advanced breast cancer
Abstract
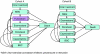
Drug therapies for advanced breast cancer in hormone-receptor-positive disease include both hormonal and chemotherapies. Current UK practice is to minimise toxicity by using sequential hormonal agents for as long as clinically appropriate. A Markov model was developed to investigate the cost effectiveness of different sequences of therapies, particularly exploring the effects of adding an additional hormonal agent, fulvestrant, to the treatment pathway. A systematic review was undertaken and a panel of seven UK oncologists validated assumptions used for treatment efficacy, treatment pathways and resources used. Fulvestrant was found to be a cost-effective treatment option when added to the treatment sequence as a second- or third-line hormonal therapy for advanced disease. For a cohort of 1000 patients, fulvestrant as a second-line hormone therapy provided an additional 47 life years and 41 quality-adjusted life years (QALYs), at an additional cost of pound 301 359. This equated to pound 6500 per life years gained and pound 7500 per QALY. When used as a third-line option, the fulvestrant arm was dominant providing an increase in health benefit of 27 QALYs for the whole cohort, at a mean overall cost reduction of pound 430 per patient. Sensitivity analyses showed these results to be robust, demonstrating that fulvestrant is an economically viable additional endocrine option in the United Kingdom for the treatment of hormone responsive advanced breast cancer.
Figures
References
-
- American Cancer society, ACS (2006) (2006) What Are the Key Statistics for Breast Cancer? http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_st...
-
- Bertelli G, Paridaens R (2006) Optimal sequence of hormonotherapy in advanced breast cancer. Curr Opin Oncol 18: 572–577 - PubMed
-
- Blum JL, Dieras V, Lo Russo PM, Horton J, Rutman O, Buzdar A, Osterwalder B (2001) Multicenter, phase II study of capecitabine in taxane-pretreated metastatic breast carcinoma patients. Cancer 92: 1759–1768 - PubMed
-
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17: 485–493 - PubMed
-
- Briggs A, Sculpher M (1998) An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 13: 397–409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical