PPARgamma agonists inhibit growth and expansion of CD133+ brain tumour stem cells
- PMID: 19018263
- PMCID: PMC2607234
- DOI: 10.1038/sj.bjc.6604786
PPARgamma agonists inhibit growth and expansion of CD133+ brain tumour stem cells
Abstract
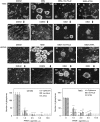
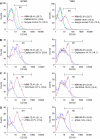
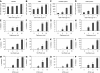
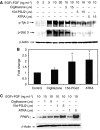
Brain tumour stem cells (BTSCs) are a small population of cells that has self-renewal, transplantation, multidrug resistance and recurrence properties, thus remain novel therapeutic target for brain tumour. Recent studies have shown that peroxisome proliferator-activated receptor gamma (PPARgamma) agonists induce growth arrest and apoptosis in glioblastoma cells, but their effects on BTSCs are largely unknown. In this study, we generated gliospheres with more than 50% CD133+ BTSC by culturing U87MG and T98G human glioblastoma cells with epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). In vitro treatment with PPARgamma agonist, 15-Deoxy-Delta(12,14)-Prostaglandin J(2) (15d-PGJ2) or all-trans retinoic acid resulted in a reversible inhibition of gliosphere formation in culture. Peroxisome proliferator-activated receptor gamma agonists inhibited the proliferation and expansion of glioma and gliosphere cells in a dose-dependent manner. Peroxisome proliferator-activated receptor gamma agonists also induced cell cycle arrest and apoptosis in association with the inhibition of EGF/bFGF signalling through Tyk2-Stat3 pathway and expression of PPARgamma in gliosphere cells. These findings demonstrate that PPARgamma agonists regulate growth and expansion of BTSCs and extend their use to target BTSCs in the treatment of brain tumour.
Figures

Similar articles
-
PPARγ agonists regulate the expression of stemness and differentiation genes in brain tumour stem cells.Br J Cancer. 2012 May 8;106(10):1702-12. doi: 10.1038/bjc.2012.161. Epub 2012 Apr 24. Br J Cancer. 2012. PMID: 22531638 Free PMC article.
-
PPARγ agonists promote oligodendrocyte differentiation of neural stem cells by modulating stemness and differentiation genes.PLoS One. 2012;7(11):e50500. doi: 10.1371/journal.pone.0050500. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185633 Free PMC article.
-
Attenuation of IFN-γ-induced B7-H1 expression by 15-deoxy-delta(12,14)-prostaglandin J2 via downregulation of the Jak/STAT/IRF-1 signaling pathway.Life Sci. 2014 Sep 1;112(1-2):82-9. doi: 10.1016/j.lfs.2014.07.021. Epub 2014 Jul 26. Life Sci. 2014. PMID: 25072357
-
15-Deoxy-delta12,14-prostaglandin J2 regulates leukemia inhibitory factor signaling through JAK-STAT pathway in mouse embryonic stem cells.Exp Cell Res. 2006 Aug 1;312(13):2538-46. doi: 10.1016/j.yexcr.2006.04.010. Epub 2006 May 5. Exp Cell Res. 2006. PMID: 16737695
-
How powerful is CD133 as a cancer stem cell marker in brain tumors?Cancer Treat Rev. 2009 Aug;35(5):403-8. doi: 10.1016/j.ctrv.2009.03.002. Epub 2009 Apr 14. Cancer Treat Rev. 2009. PMID: 19369008 Review.
Cited by
-
Tumour formation by single fibroblast growth factor receptor 3-positive rhabdomyosarcoma-initiating cells.Br J Cancer. 2009 Dec 15;101(12):2030-7. doi: 10.1038/sj.bjc.6605407. Epub 2009 Nov 3. Br J Cancer. 2009. PMID: 19888223 Free PMC article.
-
Gliomasphere marker combinatorics: multidimensional flow cytometry detects CD44+/CD133+/ITGA6+/CD36+ signature.J Cell Mol Med. 2019 Jan;23(1):281-292. doi: 10.1111/jcmm.13927. Epub 2018 Nov 22. J Cell Mol Med. 2019. PMID: 30467961 Free PMC article.
-
Brain fatty acid-binding protein and omega-3/omega-6 fatty acids: mechanistic insight into malignant glioma cell migration.J Biol Chem. 2010 Nov 19;285(47):37005-15. doi: 10.1074/jbc.M110.170076. Epub 2010 Sep 12. J Biol Chem. 2010. PMID: 20834042 Free PMC article.
-
Potential Therapeutic Effects of Thiazolidinedione on Malignant Glioma.Int J Mol Sci. 2022 Nov 4;23(21):13510. doi: 10.3390/ijms232113510. Int J Mol Sci. 2022. PMID: 36362294 Free PMC article.
-
Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer.Cells. 2022 Aug 5;11(15):2432. doi: 10.3390/cells11152432. Cells. 2022. PMID: 35954274 Free PMC article. Review.
References
-
- Annabi B, Rojas-Sutterlin S, Laflamme C, Lachambre MP, Rolland Y, Sartelet H, Béliveau R (2008) Tumor environment dictates medulloblastoma cancer stem cell expression and invasive phenotype. Mol Cancer Res 6: 907–916 - PubMed
-
- Baguma-Nibasheka M, Li AW, Murphy PR (2007) The fibroblast growth factor-2 antisense gene inhibits nuclear accumulation of FGF-2 and delays cell cycle progression in C6 glioma cells. Mol Cell Endocrinol 267: 127–136 - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760 - PubMed
-
- Blumberg B, Evans RM (1998) Orphan nuclear receptors – new ligands and new possibilities. Genes Dev 12: 3149–3155 - PubMed
-
- Bredel M, Pollack IF, Campbell JW, Hamilton RL (1997) Basic fibroblast growth factor expression as a predictor of prognosis in pediatric high-grade gliomas. Clin Cancer Res 3: 2157–2164 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

