Nanotechnology-based electrochemical sensors for biomonitoring chemical exposures
- PMID: 19018275
- PMCID: PMC2909474
- DOI: 10.1038/jes.2008.71
Nanotechnology-based electrochemical sensors for biomonitoring chemical exposures
Abstract
The coupling of dosimetry measurements and modeling represents a promising strategy for deciphering the relationship between chemical exposure and disease outcome. To support the development and implementation of biological monitoring programs, quantitative technologies for measuring xenobiotic exposure are needed. The development of portable nanotechnology-based electrochemical (EC) sensors has the potential to meet the needs for low cost, rapid, high-throughput, and ultrasensitive detectors for biomonitoring an array of chemical markers. Highly selective EC sensors capable of pM sensitivity, high-throughput and low sample requirements (<50 microl) are discussed. These portable analytical systems have many advantages over currently available technologies, thus potentially representing the next generation of biomonitoring analyzers. This paper highlights research focused on the development of field-deployable analytical instruments based on EC detection. Background information and a general overview of EC detection methods and integrated use of nanomaterials in the development of these sensors are provided. New developments in EC sensors using various types of screen-printed electrodes, integrated nanomaterials, and immunoassays are presented. Recent applications of EC sensors for assessing exposure to pesticides or detecting biomarkers of disease are highlighted to demonstrate the ability to monitor chemical metabolites, enzyme activity, or protein biomarkers of disease. In addition, future considerations and opportunities for advancing the use of EC platforms for dosimetric studies are discussed.
Figures
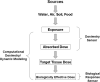

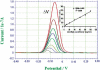
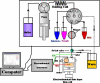



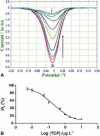

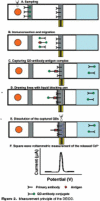


References
-
- Al-Saleh IA. Pesticides: a review article. J Environ Path Toxicol Oncol. 1994;13:151–161. - PubMed
-
- Andreescu S, Marty J-L. Twenty years research in cholinesterase biosensors: from basic research to practical applications. Biomol Engineer. 2006;23:1–15. - PubMed
-
- Angerer J, Bird MG, Burke TA, Doerrer NG, Needham L, Robison SH, et al. Strategic biomonitoring initiatives: Moving the science forward. Toxicol Sci. 2006;93(1):3–10. - PubMed
-
- Arnold EK, Beasley VR. The pharmacokinetics of chlorinated phenoxy acid herbicidnes: a literature review. Vet Hum Toxicol. 1989;31(2):121–125. - PubMed
-
- Ashley K. Developments in electrochemical sensors for occupational and environmental health applications. J Haz Mat. 2003;102:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

