Drosophila neurotrophins reveal a common mechanism for nervous system formation
- PMID: 19018662
- PMCID: PMC2586362
- DOI: 10.1371/journal.pbio.0060284
Drosophila neurotrophins reveal a common mechanism for nervous system formation
Abstract
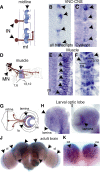
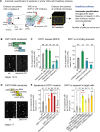
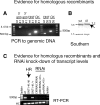
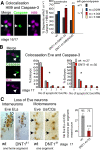
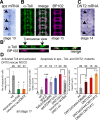
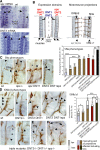
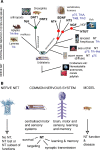
Neurotrophic interactions occur in Drosophila, but to date, no neurotrophic factor had been found. Neurotrophins are the main vertebrate secreted signalling molecules that link nervous system structure and function: they regulate neuronal survival, targeting, synaptic plasticity, memory and cognition. We have identified a neurotrophic factor in flies, Drosophila Neurotrophin (DNT1), structurally related to all known neurotrophins and highly conserved in insects. By investigating with genetics the consequences of removing DNT1 or adding it in excess, we show that DNT1 maintains neuronal survival, as more neurons die in DNT1 mutants and expression of DNT1 rescues naturally occurring cell death, and it enables targeting by motor neurons. We show that Spätzle and a further fly neurotrophin superfamily member, DNT2, also have neurotrophic functions in flies. Our findings imply that most likely a neurotrophin was present in the common ancestor of all bilateral organisms, giving rise to invertebrate and vertebrate neurotrophins through gene or whole-genome duplications. This work provides a missing link between aspects of neuronal function in flies and vertebrates, and it opens the opportunity to use Drosophila to investigate further aspects of neurotrophin function and to model related diseases.
Conflict of interest statement
Figures



References
-
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. - PubMed
-
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology. 2005;20:70–78. - PubMed
-
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. - PubMed
-
- Zweifel L. Functions and mechanisms of retrograde neurotrophin signalling. Nat Rev Neurosci. 2005;6:615–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

