Evidence for a human leucocyte antigen-DM-induced structural change in human leucocyte antigen-DObeta
- PMID: 19019088
- PMCID: PMC2712109
- DOI: 10.1111/j.1365-2567.2008.02984.x
Evidence for a human leucocyte antigen-DM-induced structural change in human leucocyte antigen-DObeta
Abstract
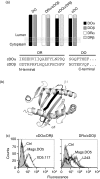
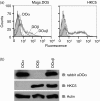
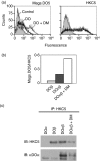
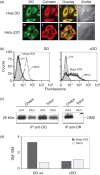
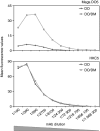
Human leucocyte antigen (HLA)-DO is a non-classical major histocompatibility complex class II molecule which modulates the function of HLA-DM and the loading of antigenic peptides on molecules such as HLA-DR. The bulk of HLA-DO associates with HLA-DM and this interaction is critical for HLA-DO egress from the endoplasmic reticulum. HLA-DM assists the early steps of HLA-DO maturation presumably through the stabilization of the interactions between the N-terminal regions of the alpha and beta chains. To evaluate a possible role for HLA-DM in influencing the conformation of HLA-DO, we made use of a monoclonal antibody, Mags.DO5, that was raised against HLA-DO/DM complexes. Using transfected cells expressing mismatched heterodimers between HLA-DR and -DO chains, we found that the epitope for Mags.DO5 is located on the DObeta chain and that Mags.DO5 reactivity was increased upon cotransfection with HLA-DM. Our results suggest that HLA-DM influences the folding of HLA-DO in the endoplasmic reticulum. A mutant HLA-DO showing reduced capacity for endoplasmic reticulum egress was better recognized by Mags.DO5 in the presence of HLA-DM. On the other hand, an HLA-DO mutant capable of endoplasmic reticulum egress on its own was efficiently recognized by Mags.DO5, irrespective of the presence of HLA-DM. Taken together, our results suggest that HLA-DM acts as a private chaperone, directly assisting the folding of HLA-DO to promote egress from the endoplasmic reticulum.
Figures
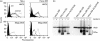
References
-
- Cresswell P. Assembly, transport, and function of MHC class-II molecules. Annu Rev Immunol. 1994;12:259–93. - PubMed
-
- Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–16. - PubMed
-
- Lotteau V, Teyton L, Peleraux A, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–5. - PubMed
-
- Roche PA, Cresswell P. Invariant chain association with HLA-DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

