Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling
- PMID: 19019093
- PMCID: PMC2712101
- DOI: 10.1111/j.1365-2567.2008.02979.x
Selective induction of the Notch ligand Jagged-1 in macrophages by soluble egg antigen from Schistosoma mansoni involves ERK signalling
Abstract
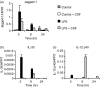
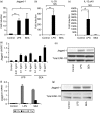
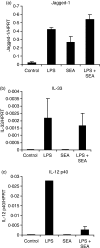
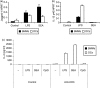
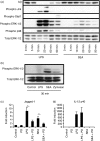
Soluble egg antigen (SEA) from the helminth Schistosoma mansoni promotes T helper type 2 (Th2) responses by modulating antigen-presenting cell function. The Jagged/Notch pathway has recently been implicated in driving Th2 development. We show here that SEA rapidly up-regulated mRNA and protein expression of the Notch ligand Jagged-1 in both murine bone marrow-derived macrophages (BMMs) and human monocyte-derived macrophages (HMDMs). Another potential Th2-promoting factor, interleukin (IL)-33, was not transcriptionally induced by SEA in BMMs. Up-regulation of Jagged-1 mRNA by SEA was also apparent in conventional dendritic cells (DCs), although the effect was less striking than in BMMs. Conversely, SEA-pulsed DCs, but not BMMs, promoted IL-4 production upon T-cell activation, suggesting that Jagged-1 induction alone is insufficient for instructing Th2 development. A comparison of the responses initiated in BMMs by SEA and the bacterial endotoxin lipopolysaccharide (LPS) revealed common activation of extracellular signal-regulated kinase-1/2 (ERK-1/2) and p38 phosphorylation, as well as induction of Jagged-1 mRNA. However, only LPS triggered IkappaB degradation, phosphorylation of c-Jun N-terminal kinase (Jnk) and signal transducer and activator of transcription 1 (Stat1) Tyr701, and IL-33 and IL-12p40 mRNA up-regulation. Inducible gene expression was modified by the presence of the macrophage growth factor colony-stimulating factor (CSF)-1, which inhibited Jagged-1 induction by SEA and LPS, but enhanced LPS-induced IL-12p40 expression. Unlike LPS, SEA robustly activated signalling in HEK293 cells expressing either Toll-like receptor 2 (TLR2) or TLR4/MD2. Pharmacological inhibition of the ERK-1/2 pathway impaired SEA- and LPS-inducible Jagged-1 expression in BMMs. Taken together, our data suggest that Jagged-1 is an ERK-dependent target of TLR signalling that has a macrophage-specific function in the response to SEA.
Figures
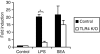

References
-
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–46. - PubMed
-
- Miyatake S, Arai N, Arai K. Chromatin remodeling and T helper subset differentiation. IUBMB Life. 2000;49:473–8. - PubMed
-
- Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

