The ribosomal stalk is required for ribosome binding, depurination of the rRNA and cytotoxicity of ricin A chain in Saccharomyces cerevisiae
- PMID: 19019145
- PMCID: PMC2637795
- DOI: 10.1111/j.1365-2958.2008.06492.x
The ribosomal stalk is required for ribosome binding, depurination of the rRNA and cytotoxicity of ricin A chain in Saccharomyces cerevisiae
Abstract
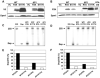
Ribosome inactivating proteins (RIPs) like ricin, pokeweed antiviral protein (PAP) and Shiga-like toxins 1 and 2 (Stx1 and Stx2) share the same substrate, the alpha-sarcin/ricin loop, but differ in their specificities towards prokaryotic and eukaryotic ribosomes. Ricin depurinates the eukaryotic ribosomes more efficiently than the prokaryotic ribosomes, while PAP can depurinate both types of ribosomes. Accumulating evidence suggests that different docking sites on the ribosome might be used by different RIPs, providing a basis for understanding the mechanism underlying their kingdom specificity. Our previous results demonstrated that PAP binds to the ribosomal protein L3 to depurinate the alpha-sarcin/ricin loop and binding of PAP to L3 was critical for its cytotoxicity. Here, we used surface plasmon resonance to demonstrate that ricin toxin A chain (RTA) binds to the P1 and P2 proteins of the ribosomal stalk in Saccharomyces cerevisiae. Ribosomes from the P protein mutants were depurinated less than the wild-type ribosomes when treated with RTA in vitro. Ribosome depurination was reduced when RTA was expressed in the DeltaP1 and DeltaP2 mutants in vivo and these mutants were more resistant to the cytotoxicity of RTA than the wild-type cells. We further show that while RTA, Stx1 and Stx2 have similar requirements for ribosome depurination, PAP has different requirements, providing evidence that the interaction of RIPs with different ribosomal proteins is responsible for their ribosome specificity.
Figures





References
-
- Ayub MJ, Smulski CR, Ma KW, Levin MJ, Shaw PC, Wong KB. The C-terminal end of P proteins mediates ribosome inactivation by trichosanthin but does not affect the pokeweed antiviral protein activity. Biochem Biophys Res Commun. 2008;369:314–319. - PubMed
-
- Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science. 2000;289:905–920. - PubMed
-
- Baykal U, Tumer NE. The C-terminus of pokeweed antiviral protein has distinct roles in transport to the cytosol, ribosome depurination and cytotoxicity. Plant J. 2007;49:995–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

