Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study
- PMID: 19019888
- PMCID: PMC2732899
- DOI: 10.1136/ard.2008.092866
Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study
Abstract
Objectives: To evaluate the safety and efficacy of 5-year, long-term tocilizumab monotherapy for patients with rheumatoid arthritis.
Methods: In an open-label, long-term extension trial following an initial 3-month randomised phase II trial, 143 of the 163 patients who participated in the initial blinded study received tocilizumab monotherapy (8 mg/kg) every 4 weeks. Concomitant therapy with non-steroidal anti-inflammatory drugs and/or oral prednisolone (10 mg daily maximum) was permitted. All patients were evaluated with American College of Rheumatology (ACR) improvement criteria, disease activity score (DAS) in 28 joints, and the European League Against Rheumatism response, as well as for safety issues.
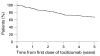
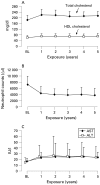
Results: 143 patients were enrolled in the open-label, long-term extension trial and 94 (66%) patients had completed 5 years as of March 2007. 32 patients (22%) withdrew from the study due to adverse events and one patient (0.7%) due to unsatisfactory response. 14 patients withdrew because of the patient's request or other reasons. The serious adverse event rate was 27.5 events per 100 patient-years, with 5.7 serious infections per 100 patient-years, based on a total tocilizumab exposure of 612 patient-years. Of the 88 patients receiving corticosteroids at baseline, 78 (88.6%) were able to decrease their corticosteroid dose and 28 (31.8%) discontinued corticosteroids. At 5 years, 79/94 (84.0%), 65/94 (69.1%) and 41/94 (43.6%) of the patients achieved ACR20, ACR50, and ACR70 improvement criteria, respectively. Remission defined as DAS28 less than 2.6 was achieved in 52/94 (55.3%) of the patients.
Conclusion: In this 5-year extension study, tocilizumab demonstrated sustained long-term efficacy and a generally good safety profile.
Conflict of interest statement
Figures

References
-
- Harris ED., Jr Rheumatoid arthritis: pathophysiology and implications for therapy. N Engl J Med 1990;322:1277–89 - PubMed
-
- Choy EHS, Isenberg DA, Garrood T, et al. Therapeutic benefit after blocking interleukin-6 activity in rheumatoid arthritis with an anti-interleukin-6 receptor monoclonal antibody. Arthritis Rheum 2002;46:3143–50 - PubMed
-
- Nishimoto N, Yoshizaki K, Maeda K, et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol 2003;30:1426–35 - PubMed
-
- Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004;50:1761–9 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

