Respiratory syncytial virus activates innate immunity through Toll-like receptor 2
- PMID: 19019963
- PMCID: PMC2620898
- DOI: 10.1128/JVI.00671-08
Respiratory syncytial virus activates innate immunity through Toll-like receptor 2
Abstract
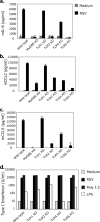
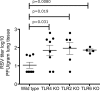
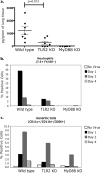
Respiratory syncytial virus (RSV) is a common cause of infection that is associated with a range of respiratory illnesses, from common cold-like symptoms to serious lower respiratory tract illnesses such as pneumonia and bronchiolitis. RSV is the single most important cause of serious lower respiratory tract illness in children <1 year of age. Host innate and acquired immune responses activated following RSV infection have been suspected to contribute to RSV disease. Toll-like receptors (TLRs) activate innate and acquired immunity and are candidates for playing key roles in the host immune response to RSV. Leukocytes express TLRs, including TLR2, TLR6, TLR3, TLR4, and TLR7, that can interact with RSV and promote immune responses following infection. Using knockout mice, we have demonstrated that TLR2 and TLR6 signaling in leukocytes can activate innate immunity against RSV by promoting tumor necrosis factor alpha, interleukin-6, CCL2 (monocyte chemoattractant protein 1), and CCL5 (RANTES). As previously noted, TLR4 also contributes to cytokine activation (L. M. Haynes, D. D. Moore, E. A. Kurt-Jones, R. W. Finberg, L. J. Anderson, and R. A. Tripp, J. Virol. 75:10730-10737, 2001, and E. A. Kurt-Jones, L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg, Nat. Immunol. 1:398-401, 2000). Furthermore, we demonstrated that signals generated following TLR2 and TLR6 activation were important for controlling viral replication in vivo. Additionally, TLR2 interactions with RSV promoted neutrophil migration and dendritic cell activation within the lung. Collectively, these studies indicate that TLR2 is involved in RSV recognition and subsequent innate immune activation.
Figures

References
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. - PubMed
-
- Applequist, S. E., R. P. Wallin, and H. G. Ljunggren. 2002. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int. Immunol. 141065-1074. - PubMed
-
- Awomoyi, A. A., P. Rallabhandi, T. I. Pollin, E. Lorenz, M. B. Sztein, M. S. Boukhvalova, V. G. Hemming, J. C. Blanco, and S. N. Vogel. 2007. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J. Immunol. 1793171-3177. - PubMed
-
- Becker, S., J. Quay, and J. Soukup. 1991. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 1474307-4312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

