Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement
- PMID: 19019965
- PMCID: PMC2620895
- DOI: 10.1128/JVI.01797-08
Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement
Abstract
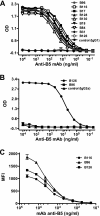
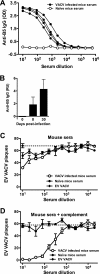
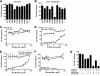
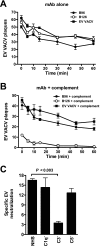
Antibody neutralization is an important component of protective immunity against vaccinia virus (VACV). Two distinct virion forms, mature virion and enveloped virion (MV and EV, respectively), possess separate functions and nonoverlapping immunological properties. In this study we examined the mechanics of EV neutralization, focusing on EV protein B5 (also called B5R). We show that neutralization of EV is predominantly complement dependent. From a panel of high-affinity anti-B5 monoclonal antibodies (MAbs), the only potent neutralizer in vitro (90% at 535 ng/ml) was an immunoglobulin G2a (IgG2a), and neutralization was complement mediated. This MAb was the most protective in vivo against lethal intranasal VACV challenge. Further studies demonstrated that in vivo depletion of complement caused a >50% loss of anti-B5 IgG2a protection, directly establishing the importance of complement for protection against the EV form. However, the mechanism of protection is not sterilizing immunity via elimination of the inoculum as the viral inoculum consisted of a purified MV form. The prevention of illness in vivo indicated rapid control of infection. We further demonstrate that antibody-mediated killing of VACV-infected cells expressing surface B5 is a second protective mechanism provided by complement-fixing anti-B5 IgG. Cell killing was very efficient, and this effector function was highly isotype specific. These results indicate that anti-B5 antibody-directed cell lysis via complement is a powerful mechanism for clearance of infected cells, keeping poxvirus-infected cells from being invisible to humoral immune responses. These findings highlight the importance of multiple mechanisms of antibody-mediated protection against VACV and point to key immunobiological differences between MVs and EVs that impact the outcome of infection.
Figures


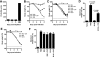


References
-
- Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. Hirao, S. N. Isaacs, B. Moss, R. J. Eisenberg, and G. H. Cohen. 2005. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 796260-6271. - PMC - PubMed
-
- Aldaz-Carroll, L., J. C. Whitbeck, M. Ponce de Leon, H. Lou, L. K. Pannell, J. Lebowitz, C. Fogg, C. L. White, B. Moss, G. H. Cohen, and R. J. Eisenberg. 2005. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology 34159-71. - PubMed
-
- Aldaz-Carroll, L., Y. Xiao, J. C. Whitbeck, M. Ponce de Leon, H. Lou, M. Kim, J. Yu, E. L. Reinherz, S. N. Isaacs, R. J. Eisenberg, and G. H. Cohen. 2007. Major neutralizing sites on vaccinia virus glycoprotein B5 are exposed differently on variola virus ortholog B6. J. Virol. 818131-8139. - PMC - PubMed
-
- Amanna, I. J., M. K. Slifka, and S. Crotty. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211320-337. - PubMed
-
- Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325425-431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

