Hepatitis C virus neuroinvasion: identification of infected cells
- PMID: 19019968
- PMCID: PMC2620915
- DOI: 10.1128/JVI.01890-08
Hepatitis C virus neuroinvasion: identification of infected cells
Abstract
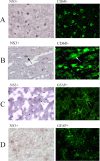
Hepatitis C virus (HCV) infection often is associated with cognitive dysfunction and depression. HCV sequences and replicative forms were detected in autopsy brain tissue and cerebrospinal fluid from infected patients, suggesting direct neuroinvasion. However, the phenotype of cells harboring HCV in brain remains unclear. We studied autopsy brain tissue from 12 HCV-infected patients, 6 of whom were coinfected with human immunodeficiency virus. Cryostat sections of frontal cortex and subcortical white matter were stained with monoclonal antibodies specific for microglia/macrophages (CD68), oligodendrocytes (2',3'-cyclic nucleotide 3'-phosphodiesterase), astrocytes (glial fibrillary acidic protein [GFAP]), and neurons (neuronal-specific nuclear protein); separated by laser capture microscopy (LCM); and tested for the presence of positive- and negative-strand HCV RNA. Sections also were stained with antibodies to viral nonstructural protein 3 (NS3), separated by LCM, and phenotyped by real-time PCR. Finally, sections were double stained with antibodies specific for the cell phenotype and HCV NS3. HCV RNA was detected in CD68-positive cells in eight patients, and negative-strand HCV RNA, which is a viral replicative form, was found in three of these patients. HCV RNA also was found in astrocytes from three patients, but negative-strand RNA was not detected in these cells. In double immunostaining, 83 to 95% of cells positive for HCV NS3 also were CD68 positive, while 4 to 29% were GFAP positive. NS3-positive cells were negative for neuron and oligodendrocyte phenotypic markers. In conclusion, HCV infects brain microglia/macrophages and, to a lesser extent, astrocytes. Our findings could explain the biological basis of neurocognitive abnormalities in HCV infection.
Figures

Similar articles
-
Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication.J Virol. 2002 Jan;76(2):600-8. doi: 10.1128/jvi.76.2.600-608.2002. J Virol. 2002. PMID: 11752151 Free PMC article.
-
Activation of brain macrophages/microglia cells in hepatitis C infection.Gut. 2010 Oct;59(10):1394-400. doi: 10.1136/gut.2009.199356. Epub 2010 Jul 30. Gut. 2010. PMID: 20675697
-
Detection of hepatitis C virus sequences in brain tissue obtained in recurrent hepatitis C after liver transplantation.Liver Transpl. 2002 Nov;8(11):1014-9. doi: 10.1053/jlts.2002.36393. Liver Transpl. 2002. PMID: 12424714
-
Emerging evidence of hepatitis C virus neuroinvasion.AIDS. 2005 Oct;19 Suppl 3:S140-4. doi: 10.1097/01.aids.0000192083.41561.00. AIDS. 2005. PMID: 16251811 Review.
-
Hepatitis C virus and the brain.J Viral Hepat. 2012 May;19(5):301-6. doi: 10.1111/j.1365-2893.2012.01591.x. J Viral Hepat. 2012. PMID: 22497808 Review.
Cited by
-
Brain magnetic resonance spectroscopy and cognitive impairment in chronic hepatitis C patients.Egypt J Neurol Psychiatr Neurosurg. 2018;54(1):43. doi: 10.1186/s41983-018-0046-7. Epub 2018 Dec 20. Egypt J Neurol Psychiatr Neurosurg. 2018. PMID: 30613130 Free PMC article.
-
Association between dementia and hepatitis B and C virus infection.Medicine (Baltimore). 2021 Jul 23;100(29):e26476. doi: 10.1097/MD.0000000000026476. Medicine (Baltimore). 2021. PMID: 34398003 Free PMC article.
-
Hepatitis C virus infection, and neurological and psychiatric disorders - A review.J Adv Res. 2017 Mar;8(2):139-148. doi: 10.1016/j.jare.2016.09.005. Epub 2016 Sep 19. J Adv Res. 2017. PMID: 28149649 Free PMC article. Review.
-
Vasculitis: determinants of disease patterns.Nat Rev Rheumatol. 2014 Aug;10(8):454-62. doi: 10.1038/nrrheum.2014.89. Epub 2014 Jun 17. Nat Rev Rheumatol. 2014. PMID: 24934189 Review.
-
Hepatitis C virus infects the endothelial cells of the blood-brain barrier.Gastroenterology. 2012 Mar;142(3):634-643.e6. doi: 10.1053/j.gastro.2011.11.028. Epub 2011 Dec 1. Gastroenterology. 2012. PMID: 22138189 Free PMC article.
References
-
- Adair, D. M., M. Radkowski, J. Jablonska, A. Pawelczyk, J. Wilkinson, J. Rakela, and T. Laskus. 2005. Differential display analysis of gene expression in brains from hepatitis C-infected patients. AIDS 19(Suppl. 3)S145-S150. - PubMed
-
- Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120512-524. - PubMed
-
- Barkhuizen, A., H. R. Rosen, S. Wolf, K. Flora, K. Benner, and R. M. Bennett. 1999. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: a report of 239 hepatology clinic patients. Am. J. Gastroenterol. 941355-1360. - PubMed
-
- Capuron, L., and R. Dantzer. 2003. Cytokines and depression: the need for a new paradigm. Brain Behav. Immun. 17(Suppl. 1)S119-S124. - PubMed
-
- Caussin-Schwemling, C., C. Schmitt, and F. Stoll-Keller. 2001. Study of the infection of human blood derived monocyte/macrophages with hepatitis C virus in vitro. J. Med. Virol. 6514-22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

