Imatinib suppresses cryoglobulinemia and secondary membranoproliferative glomerulonephritis
- PMID: 19020005
- PMCID: PMC2615727
- DOI: 10.1681/ASN.2008010036
Imatinib suppresses cryoglobulinemia and secondary membranoproliferative glomerulonephritis
Abstract
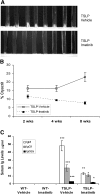
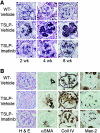
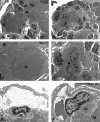
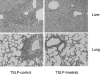
Imatinib is a receptor tyrosine kinase inhibitor that blocks the activity of c-Abl, c-Kit, and PDGF receptors. We tested the protective effects of imatinib in thymic stromal lymphopoietin transgenic mice, a model of cryoglobulinemia and associated membranoproliferative glomerulonephritis (MPGN), in which some glomerular manifestations likely result from PDGF receptor activation. Surprising, administration of imatinib beginning at weaning suppressed production of cryoglobulin, attenuating both the renal injury and systemic features of cryoglobulinemia. Flow cytometry suggested that inhibition of B cell development in the bone marrow likely caused the reduction in cryoglobulin production. In addition, administration of imatinib to thymic stromal lymphopoietin transgenic mice with established MPGN also diminished cryoglobulin production and reversed the renal and systemic lesions. These data suggest that treatment with imatinib may be a novel therapeutic approach for cryoglobulinemia and MPGN in humans.
Figures

References
-
- Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A: A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 22: 321–328, 1994 - PubMed
-
- Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG: Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol 162: 677–683, 1999 - PubMed
-
- Ray RJ, Furlonger C, Williams DE, Paige CJ: Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol 26: 10–16, 1996 - PubMed
-
- Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF: Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6: 1047–1053, 2005 - PubMed
-
- Ziegler SF, Liu YJ: Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol 7: 709–714, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

