Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration
- PMID: 19020025
- PMCID: PMC2819191
- DOI: 10.1523/JNEUROSCI.3918-08.2008
Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration
Abstract
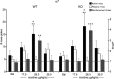
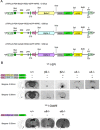
The identification of the molecular mechanisms involved in nicotine addiction and its cognitive consequences is a worldwide priority for public health. Novel in vivo paradigms were developed to match this aim. Although the beta2 subunit of the neuronal nicotinic acetylcholine receptor (nAChR) has been shown to play a crucial role in mediating the reinforcement properties of nicotine, little is known about the contribution of the different alpha subunit partners of beta2 (i.e., alpha4 and alpha6), the homo-pentameric alpha7, and the brain areas other than the ventral tegmental area (VTA) involved in nicotine reinforcement. In this study, nicotine (8.7-52.6 microg free base/kg/inf) self-administration was investigated with drug-naive mice deleted (KO) for the beta2, alpha4, alpha6 and alpha7 subunit genes, their wild-type (WT) controls, and KO mice in which the corresponding nAChR subunit was selectively re-expressed using a lentiviral vector (VEC mice). We show that WT mice, beta2-VEC mice with the beta2 subunit re-expressed exclusively in the VTA, alpha4-VEC mice with selective alpha4 re-expression in the VTA, alpha6-VEC mice with selective alpha6 re-expression in the VTA, and alpha7-KO mice promptly self-administer nicotine intravenously, whereas beta2-KO, beta2-VEC in the substantia nigra, alpha4-KO and alpha6-KO mice do not respond to nicotine. We thus define the necessary and sufficient role of alpha4beta2- and alpha6beta2-subunit containing nicotinic receptors (alpha4beta2*- and alpha6beta2*-nAChRs), but not alpha7*-nAChRs, present in cell bodies of the VTA, and their axons, for systemic nicotine reinforcement in drug-naive mice.
Figures




References
-
- Alderson HL, Latimer MP, Winn P. Intravenous self-administration of nicotine is altered by lesions of the posterior, but not anterior, pedunculopontine tegmental nucleus. Eur J Neurosci. 2006;23:2169–2175. - PubMed
-
- Balfour DJ. The neurobiology of tobacco dependence: a commentary. Respiration. 2002;69:7–11. - PubMed
-
- Benowitz NL. Compensatory smoking of low-yield cigarettes. In: Burns DM, Shopland DR, Benowitz NL, editors. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: US National Institutes of Health, National Cancer Institute; 2001. pp. 39–63.
-
- Benowitz NL, Jacob P., 3rd Nicotine renal excretion rate influences nicotine intake during cigarette smoking. J Pharmacol Exp Ther. 1985;234:153–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
