A bifunctional kinase-phosphatase in bacterial chemotaxis
- PMID: 19020080
- PMCID: PMC2587623
- DOI: 10.1073/pnas.0808010105
A bifunctional kinase-phosphatase in bacterial chemotaxis
Abstract
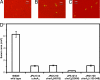
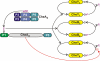
Phosphorylation-based signaling pathways employ dephosphorylation mechanisms for signal termination. Histidine to aspartate phosphosignaling in the two-component system that controls bacterial chemotaxis has been studied extensively. Rhodobacter sphaeroides has a complex chemosensory pathway with multiple homologues of the Escherichia coli chemosensory proteins, although it lacks homologues of known signal-terminating CheY-P phosphatases, such as CheZ, CheC, FliY or CheX. Here, we demonstrate that an unusual CheA homologue, CheA(3), is not only a phosphodonor for the principal CheY protein, CheY(6), but is also is a specific phosphatase for CheY(6)-P. This phosphatase activity accelerates CheY(6)-P dephosphorylation to a rate that is comparable with the measured stimulus response time of approximately 1 s. CheA(3) possesses only two of the five domains found in classical CheAs, the Hpt (P1) and regulatory (P5) domains, which are joined by a 794-amino acid sequence that is required for phosphatase activity. The P1 domain of CheA(3) is phosphorylated by CheA(4), and it subsequently acts as a phosphodonor for the response regulators. A CheA(3) mutant protein without the 794-amino acid region lacked phosphatase activity, retained phosphotransfer function, but did not support chemotaxis, suggesting that the phosphatase activity may be required for chemotaxis. Using a nested deletion approach, we showed that a 200-amino acid segment of CheA(3) is required for phosphatase activity. The phosphatase activity of previously identified nonhybrid histidine protein kinases depends on the dimerization and histidine phosphorylation (DHp) domains. However, CheA(3) lacks a DHp domain, suggesting that its phosphatase mechanism is different from that of other histidine protein kinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. - PubMed
-
- Perego M, et al. Multiple protein-aspartate phosphatases provide a mechanism for the integration of diverse signals in the control of development in B. subtilis. Cell. 1994;79:1047–1055. - PubMed
-
- Hess JF, Oosawa K, Kaplan N, Simon MI. Phosphorylation of three proteins in the signalling pathway of bacterial chemotaxis. Cell. 1988;53:79–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

