8-Oxoguanine-mediated transcriptional mutagenesis causes Ras activation in mammalian cells
- PMID: 19020090
- PMCID: PMC2596238
- DOI: 10.1073/pnas.0806464105
8-Oxoguanine-mediated transcriptional mutagenesis causes Ras activation in mammalian cells
Abstract
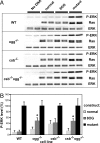
8-Oxoguanine (8OG) is efficiently bypassed by RNA polymerases in vitro and in bacterial cells in vivo, leading to mutant transcripts by directing incorporation of an incorrect nucleotide during transcription. Such transcriptional mutagenesis (TM) may produce a pool of mutant proteins. In contrast, transcription-coupled repair safeguards against DNA damage, contingent upon the ability of lesions to arrest elongating RNA polymerase. In mammalian cells, the Cockayne syndrome B protein (Csb) mediates transcription-coupled repair, and its involvement in the repair of 8OG is controversial. The DNA glycosylase Ogg1 initiates base excision repair of 8OG, but its influence on TM is unknown. We have developed a mammalian system for TM in congenic mouse embryonic fibroblasts (MEFs), either WT or deficient in Ogg1 (ogg(-/-)), Csb (csb(-/-)), or both. This system uses expression of the Ras oncogene in which an 8OG replaces guanine in codon 61. Repair of 8OG restores the WT sequence; however, bypass and misinsertion opposite this lesion during transcription leads to a constitutively active mutant Ras protein and activation of downstream signaling events, including increased phosphorylation of ERK kinase. Upon transfection of MEFs with replication-incompetent 8OG constructs, we observed a marked increase in phospho-ERK in ogg(-/-) and csb(-/-)ogg(-/-) cells at 6 h, indicating persistence of the lesion and the occurrence of TM. This effect is absent in WT and csb(-/-) cells, suggesting rapid repair. These studies provide evidence that 8OG causes TM in mammalian cells, leading to a phenotypic change with important implications for the role of TM in tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Friedberg EC. DNA damage and repair. Nature. 2003;421:436–440. - PubMed
-
- Nouspikel T, Hanawalt PC. DNA repair in terminally differentiated cells. DNA Repair (Amst) 2002;1:59–75. - PubMed
-
- Bohr VA, Smith CA, Okumoto DS, Hanawalt PC. DNA repair in an active gene: Removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985;40:359–369. - PubMed
-
- Fousteri M, Mullenders LH. Transcription-coupled nucleotide excision repair in mammalian cells: Molecular mechanisms and biological effects. Cell Res. 2008;18:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

